 [JEE MAIN Held on 09-01-2020 Morning]
[JEE MAIN Held on 09-01-2020 Morning]
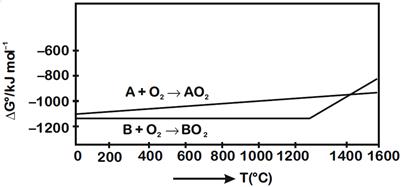
A) \[>\text{ }1400{}^\circ C\]
B) \[>\text{ }1200{}^\circ C\] but \[<\text{ }1400{}^\circ C\]
C) \[<\text{ }1200{}^\circ C\]
D) \[<\text{ }1400{}^\circ C\]
Correct Answer: A
Solution :
[a] Reduction of \[B{{O}_{2}}\] using A \[B{{O}_{2}}+A\to B+A{{O}_{2}}\] From given diagram, it is clear that, \[\Delta G{}^\circ \]of required reaction will be negative when \[T\text{ }>\text{ }1400{}^\circ C\]You need to login to perform this action.
You will be redirected in
3 sec
