A) \[{{X}_{2}}Y,\text{ }2\times {{10}^{9}}\text{ }{{M}^{3}}\]
B) \[X{{Y}_{2}},\text{ }4\times {{10}^{9}}\text{ }{{M}^{3}}\]
C) \[X{{Y}_{2}},\text{ }1\times {{10}^{9}}\text{ }{{M}^{3}}\]
D) \[XY,2\times {{10}^{6}}\text{ }{{M}^{3}}\]
Correct Answer: B
Solution :
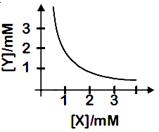
[b] From the given curve, if \[\left[ X \right]=1\text{ }mM\text{ }then\left[ Y \right]=2\text{ }mM\] \[\therefore \]Salt is \[X{{Y}_{2}}\] \[{{K}_{sp}}=\left[ X \right]{{\left[ Y \right]}^{2}}=\left( {{10}^{3}} \right){{\left( 2\times {{10}^{3}} \right)}^{2}}=4\times {{10}^{9}}\text{ }{{M}^{3}}\]You need to login to perform this action.
You will be redirected in
3 sec
