| A graph of vapour pressure and temperature for three different liquids X, Y, and Z is shown below |
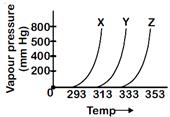 |
| The following inferences are made |
| (A) X has higher intermolecular interactions compared to Y. |
| (B) X has lower intermolecular interactions compared to Y |
| (C) Z has lower intermolecular interactions compared to Y. |
| The correct inferences is/are |
A) (B)
B) (C)
C) (A) and (C)
D) (A)
Correct Answer: A
Solution :
Vapour pressure of a liquid at a given temperature is inversely proportional to intermolecular force of attraction. At the same temperature, vapour pressure of X is higher than that of Y. Therefore (X) has lower intermolecular interactions compared to Y. Statement is correct.You need to login to perform this action.
You will be redirected in
3 sec
