A) 90 J
B) 1 J
C) 99 J
D) 100 J
Correct Answer: A
Solution :
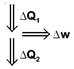 \[\frac{\Delta {{Q}_{1}}-\Delta {{Q}_{2}}}{\Delta {{Q}_{1}}}=\frac{1}{10}=\frac{\Delta w}{\Delta {{Q}_{1}}}\] \[\Rightarrow \frac{1}{10}=\frac{\Delta w}{\Delta {{Q}_{1}}}\] \[\therefore \,\,\,\,\,\,\,\,\Delta {{Q}_{1}}=\Delta w\times 10=100\,\,J\] So, \[\Delta {{Q}_{1}}-\Delta {{Q}_{2}}=\Delta w\] \[\Rightarrow \,\,\,\,\,\,100-10=\Delta {{Q}_{2}}=90\,\,J\]
\[\frac{\Delta {{Q}_{1}}-\Delta {{Q}_{2}}}{\Delta {{Q}_{1}}}=\frac{1}{10}=\frac{\Delta w}{\Delta {{Q}_{1}}}\] \[\Rightarrow \frac{1}{10}=\frac{\Delta w}{\Delta {{Q}_{1}}}\] \[\therefore \,\,\,\,\,\,\,\,\Delta {{Q}_{1}}=\Delta w\times 10=100\,\,J\] So, \[\Delta {{Q}_{1}}-\Delta {{Q}_{2}}=\Delta w\] \[\Rightarrow \,\,\,\,\,\,100-10=\Delta {{Q}_{2}}=90\,\,J\]
You need to login to perform this action.
You will be redirected in
3 sec
