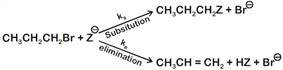
 where,
where, 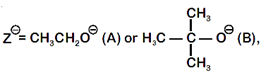 \[{{k}_{s}}\] and \[{{k}_{e}}\], are, respectively, the rate constants for substitution and elimination, and \[\mu =\frac{{{k}_{s}}}{{{k}_{e}}}\]the correct option is _________ . [JEE MAIN Held on 07-01-2020 Evening]
\[{{k}_{s}}\] and \[{{k}_{e}}\], are, respectively, the rate constants for substitution and elimination, and \[\mu =\frac{{{k}_{s}}}{{{k}_{e}}}\]the correct option is _________ . [JEE MAIN Held on 07-01-2020 Evening]
A) \[{{\mu }_{B}}>{{\mu }_{A}}\] and \[{{k}_{e}}(A)>{{k}_{e}}(B)\]
B) \[{{\mu }_{A}}>{{\mu }_{B}}\] and \[{{k}_{e}}(A)>{{k}_{e}}(B)\]
C) \[{{\mu }_{B}}>{{\mu }_{A}}\] and \[{{k}_{e}}(A)>{{k}_{e}}(B)\]
D) \[{{\mu }_{A}}>{{\mu }_{B}}\] and \[{{k}_{e}}(A)>{{k}_{e}}(B)\]
Correct Answer: D
Solution :
[d]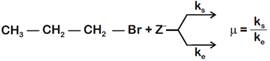 when \[{{Z}^{\Theta }}=C{{H}_{3}}C{{H}_{2}}{{O}^{-}}(A)\]
when \[{{Z}^{\Theta }}=C{{H}_{3}}C{{H}_{2}}{{O}^{-}}(A)\] You need to login to perform this action.
You will be redirected in
3 sec
