A) \[PC{{l}_{5}},I{{F}_{5}},Xe{{O}_{2}}{{F}_{2}}\]
B) \[B{{F}_{3}},PC{{l}_{3}},Xe{{O}_{3}}\]
C) \[Cl{{F}_{3}},XeO{{F}_{2}},XeF_{3}^{+}\]
D) \[S{{F}_{4}},Xe{{F}_{4}},CC{{l}_{4}}\]
Correct Answer: C
Solution :
\[Cl{{F}_{3}},XeO{{F}_{2}}\And XeF_{3}^{+}\]are \[s{{p}^{3}}d\]hybridized with 2 lone pair e's, hence all have (T-shape) identical shape.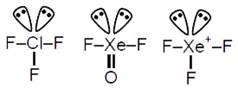
You need to login to perform this action.
You will be redirected in
3 sec
