A)
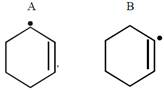
B)
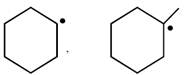
C)
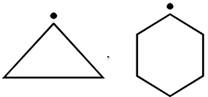
D) \[P{{h}_{3}}{{C}^{\bullet }},{{(C{{H}_{3}})}_{3}}{{C}^{\bullet }}\]
Correct Answer: D
Solution :
\[P{{h}_{3}}\overset{.}{\mathop{C}}\,\]is more stable than \[{{(C{{H}_{3}})}_{3}}\overset{.}{\mathop{C}}\,\]because resonance stabilisation effect in \[P{{h}_{3}}\overset{.}{\mathop{C}}\,\] is more pronounced as compared to hyper conjugation stabilization effect in \[{{(C{{H}_{3}})}_{3}}\overset{.}{\mathop{C}}\,\]overall stability order among free radical is : Triphenylmethyl> benzyl >allyl> tertiary alkyl > secondary > primary > methyl > vinylYou need to login to perform this action.
You will be redirected in
3 sec
