A)
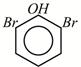
B)
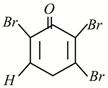
C)
![]()
D)
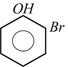
Correct Answer: A
Solution :
Phenol has activating (electron releasing) \[-\text{OH}\]group and bromine water supplies \[\text{B}{{\text{r}}^{+}}\]ion easily, hence under such conditions reaction does not stop at monobromo or dibromo stage but a fully brominated (2,4,6,-tribromophenol) compound is the final product.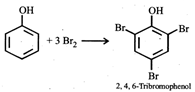
You need to login to perform this action.
You will be redirected in
3 sec
