 JEE Main Online Paper (Held On 19 May 2012)
JEE Main Online Paper (Held On 19 May 2012)
A) one
B) zero
C) two
D) three
Correct Answer: B
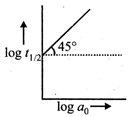
Solution :
Plot given is for zero order reaction.You need to login to perform this action.
You will be redirected in
3 sec
