 JEE Main Online Paper (Held On 19 April 2016)
JEE Main Online Paper (Held On 19 April 2016)
A) \[-C{{H}_{3}}\]
B) \[-OC{{H}_{3}}\]
C) \[-COC{{H}_{3}}\]
D) \[-C{{H}_{2}}OH\]
Correct Answer: C
Solution :
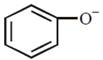
Electron withdrawing group stabilises the benzene ring due to delocalisation of charge. \[-C{{H}_{3}}\]and \[-C{{H}_{2}}OH\]are electron donating group and hence decrease the stability of benzene ring \[-OC{{H}_{3}}\] is weaker electron withdrawing group than \[-COC{{H}_{3}}.\] Hence \[-COC{{H}_{3}}\] group more stabilize the phenoxide ion at p-position.You need to login to perform this action.
You will be redirected in
3 sec
