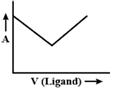
A)
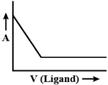
B)
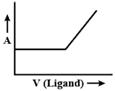
C)
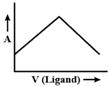
D)
Correct Answer: C
Solution :
Initially volume of ligand increases then ligand combine with metal and form a complex, so when we add ligand it will not affect absorbed light. After a period of time, when all metals are consumed then complex will not be formed so, only ligand volume will increase which will result in the increase of absorbed light.You need to login to perform this action.
You will be redirected in
3 sec
