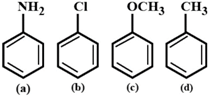
A) \[(b)<(a)<(c)<(d)\]
B) \[(b)<(a)<(d)<(c)\]
C) \[(a)<(b)<(c)<(d)\]
D) \[(a)<(b)<(d)<(c)\]
Correct Answer: B
Solution :
Nitration is electrophilic aromatic substitution reaction. Methoxy and amino groups are strongly activating groups. Methyl group is weakly activating group. Since among methyl and methoxy group, methoxy group is more reactive than methyl group, (c) is more reactive than (d). Even-though amino group is strongly activating group, it gets protonated in presence of acid) to form anilinium ion which is strongly deactivating. Hence, (a) is less reactive than (c) and (d). Chloro group is deactivating group. so (b) has least reactivity. Note: The activating groups increases the electron density on benzene ring and increases the rate of electrophilic aromatic substitution reaction. The deactivating groups decreases the electron density on benzene ring and decreases the rate of electrophilic aromatic substitution reaction.You need to login to perform this action.
You will be redirected in
3 sec
