A) \[{{[Fe{{(phen)}_{3}}]}^{2+}}\]
B) \[{{[Zn{{(phen)}_{3}}]}^{2+}}\]
C) \[{{[Ni{{(phen)}_{3}}]}^{2+}}\]
D) \[{{[Co{{(phen)}_{3}}]}^{2+}}\]
Correct Answer: A
Solution :
 symmetrical bidentate ligand.
symmetrical bidentate ligand. 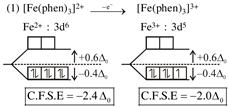 By oxidation of \[F{{e}^{2+}}\]into \[F{{e}^{3+}},\]the CFSE value decrease.
By oxidation of \[F{{e}^{2+}}\]into \[F{{e}^{3+}},\]the CFSE value decrease. 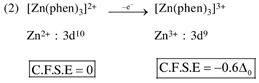 By oxidation of \[Z{{n}^{2+}}\]into \[Z{{n}^{3+}},\]the CFSE value increase.
By oxidation of \[Z{{n}^{2+}}\]into \[Z{{n}^{3+}},\]the CFSE value increase. 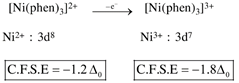 by oxidation of \[N{{i}^{2+}}\]into \[N{{i}^{3+}},\]the CFSE value increase.
by oxidation of \[N{{i}^{2+}}\]into \[N{{i}^{3+}},\]the CFSE value increase. 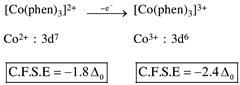 by oxidation of \[C{{o}^{2+}}\]into \[C{{o}^{3+}},\]the CFSE value increase.
by oxidation of \[C{{o}^{2+}}\]into \[C{{o}^{3+}},\]the CFSE value increase.
You need to login to perform this action.
You will be redirected in
3 sec
