A) \[{{[Co{{F}_{6}}]}^{3-}}\]
B) \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{2+}}\]
C) \[{{[Mn{{(CN)}_{6}}]}^{4-}}\]
D) \[{{[Fe{{F}_{6}}]}^{3-}}\]
Correct Answer: D
Solution :
\[{{\left[ Fe{{F}_{6}} \right]}^{3-}}\]oxidiation state of \[Fe=+3\] \[F{{e}^{+3}}=\left[ Ar \right]3{{d}^{5}},{{F}^{-}}\] is weak field Ligand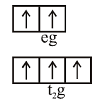
You need to login to perform this action.
You will be redirected in
3 sec
