A) dissociation
B) association
C) partial ionization
D) complex formation
Correct Answer: B
Solution :
Molar mass of acetic acid in benzene using freezing point depression is affected by association.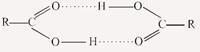
You need to login to perform this action.
You will be redirected in
3 sec
