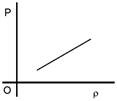
A)
B)
C)
D)
Correct Answer: A
Solution :
\[p=\frac{PM}{RT}\] \[P=\frac{\rho RT}{M}\] \[P\propto \rho \]You need to login to perform this action.
You will be redirected in
3 sec
