| (en = ethane | 1, 2 | diamine, ox = oxalate) |
A) \[[Pt(en)C{{l}_{2}}]\]
B) \[{{[Cr{{(en)}_{2}}(ox)]}^{+}}\]
C) \[[Zn(en)C{{l}_{2}}]\]
D) \[{{[Pt{{(en)}_{2}}C{{l}_{2}}]}^{2+}}\]
Correct Answer: D
Solution :
[a]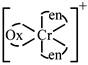 no trans isomer possible [c]
no trans isomer possible [c] 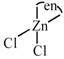 \[s{{p}^{3}}\] hybridized so no trans possible [d]
\[s{{p}^{3}}\] hybridized so no trans possible [d]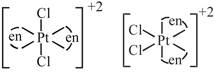 trans and cis both are possible
trans and cis both are possible
You need to login to perform this action.
You will be redirected in
3 sec
