| [a] saline hydrides produce \[{{H}_{2}}\]gas when reacted with \[{{H}_{2}}O\]. |
| [b] reaction of \[LiA{{H}_{4}}\]with \[B{{F}_{3}}\]leads to \[{{B}_{2}}{{H}_{6}}.\] |
| [c] \[P{{H}_{3}}\] and \[C{{H}_{4}}\] are electron - rich and electron precise hydrides, respectively. |
| [d] \[HF\]and \[C{{H}_{4}}\]are called as molecular hydrides. |
A) [c] and [d] only
B) [a], [b] and [c] only
C) [a], [b], [c] and [d]
D) [a], [c] and [d] only
Correct Answer: C
Solution :
[a] \[\underset{Ionichydride/salinehydride}{\mathop{\overset{\oplus }{\mathop{M}}\,\overset{\Theta }{\mathop{H}}\,+HOH}}\,\xrightarrow[{}]{{}}MOH+{{H}_{2}}\] [b]\[4B{{F}_{3}}+3LiAl{{H}_{4}}\xrightarrow[{}]{{}}2{{B}_{2}}{{H}_{6}}+3LiF+3Al{{F}_{3}}\] [c]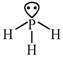 \[\to \]phosphorous is electron rich hydride due to presence of lone pair
\[\to \]phosphorous is electron rich hydride due to presence of lone pair 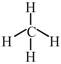 \[\to \]It is electron precise hydride. [d] \[HF\And C{{H}_{4}}\]are molecular hydride due to they are covalent molecules.
\[\to \]It is electron precise hydride. [d] \[HF\And C{{H}_{4}}\]are molecular hydride due to they are covalent molecules.
You need to login to perform this action.
You will be redirected in
3 sec
