A) \[\Delta {{Q}_{A}}=\Delta {{Q}_{B}};\Delta {{U}_{A}}=\Delta {{U}_{B}}\]
B) \[\Delta {{Q}_{A}}>\Delta {{Q}_{B}};\Delta {{U}_{A}}=\Delta {{U}_{B}}\]
C) \[\Delta {{Q}_{A}}>\Delta {{Q}_{B}};\Delta {{U}_{A}}>\Delta {{U}_{B}}\]
D) \[\Delta {{Q}_{A}}<\Delta {{Q}_{B}};\Delta {{U}_{A}}<\Delta {{U}_{B}}\]
Correct Answer: B
Solution :
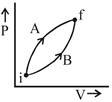 Initial and final states for both the processes are same. \[\therefore \]\[\Delta {{U}_{A}}=\Delta {{U}_{B}}\] Work done during process A is greater than in process B. By First Law of thermodynamics \[\Delta Q=\Delta U+W\]\[\Rightarrow \]\[\Delta {{Q}_{A}}>\Delta {{Q}_{B}}\]
Initial and final states for both the processes are same. \[\therefore \]\[\Delta {{U}_{A}}=\Delta {{U}_{B}}\] Work done during process A is greater than in process B. By First Law of thermodynamics \[\Delta Q=\Delta U+W\]\[\Rightarrow \]\[\Delta {{Q}_{A}}>\Delta {{Q}_{B}}\]
You need to login to perform this action.
You will be redirected in
3 sec
