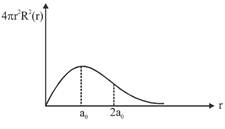
A) The electron can be found at a distance \[2{{a}_{0}}\]from the nucleus
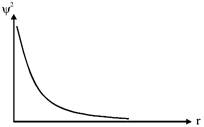
B) The probability density of finding the electron is maximum at the nucleus.
C) The magnitude of potential energy is double that of its kinetic energy on an average.
D) The total energy of the electron is maximum when it is at a distance \[{{a}_{0}}\] from the nucleus.
Correct Answer: D
Solution :
You need to login to perform this action.
You will be redirected in
3 sec
