| [A] | [B] |
| [C] | [D]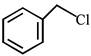 |
A) \[\left( B \right)<\left( C \right)<\left( D \right)<\left( A \right)\]
B) \[\left( A \right)<\left( B \right)<\left( D \right)<\left( C \right)\]
C) \[\left( B \right)<\left( A \right)<\left( D \right)<\left( C \right)\]
D) \[\left( B \right)<\left( C \right)<\left( A \right)<\left( D \right)\]
Correct Answer: C
Solution :
\[{{S}_{N}}1\]Reactivity order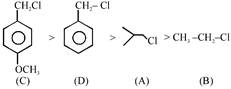 \[Order\text{ }C>D>A>B\]
\[Order\text{ }C>D>A>B\]
You need to login to perform this action.
You will be redirected in
3 sec
