A) 45%
B) 65%
C) 90%
D) 75%
Correct Answer: C
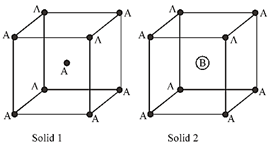
Solution :
\[p.f.=\frac{{{\left( {{z}_{eff}}\times \frac{4}{3}\pi r_{A}^{3} \right)}_{A}}+{{\left( {{z}_{eff}}\times \frac{4}{3}\pi r_{B}^{3} \right)}_{B}}}{{{a}^{3}}}\] \[2({{r}_{A}}+{{r}_{B}})=\sqrt{3}a\] \[\Rightarrow \]\[2({{r}_{A}}+2{{r}_{A}})=\sqrt{3}a\]\[\Rightarrow \]\[2\sqrt{3}{{r}_{A}}=a\] \[\Rightarrow \]\[p.f.=\frac{1\times \frac{4}{3}\pi r_{A}^{3}+\frac{4}{3}\pi \left( 8r_{A}^{3} \right)}{8\times 3\sqrt{3}\,\,{{r}_{A}}^{3}}=\frac{9\times \frac{4}{3}\pi }{8\times 3\sqrt{3}}=\frac{\pi }{2\sqrt{3}}\] p. efficiency\[=\frac{\pi }{2\sqrt{3}}\times 100\simeq 90%\]You need to login to perform this action.
You will be redirected in
3 sec
