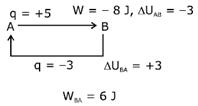
A) 6 J of the work will be done by the gas
B) 6 J of the work will be done by the surrounding on gas.
C) 10 J of the work will be done by the surrounding on gas.
D) 10 J of the work will be done by the gas
Correct Answer: B
Solution :
You need to login to perform this action.
You will be redirected in
3 sec
