A) High ionization enthalpy of fluorine
B) Smaller size of chlorine atom
C) Smaller size of fluorine atom
D) Bigger size of \[2p\]orbital of fluorine
Correct Answer: C
Solution :
Due to smaller size and high repulsive force within the outermost orbit of fluorine electron will feel less repulsion.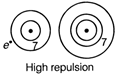
You need to login to perform this action.
You will be redirected in
3 sec
