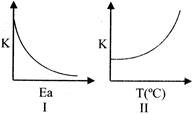
 Choose the correct option:
[JEE Main Online Paper (Held On 10-Jan-2019 Morning]
Choose the correct option:
[JEE Main Online Paper (Held On 10-Jan-2019 Morning]
A) I is right but II is wrong
B) Both I and II are correct
C) Both I and II are wrong
D) I is wrong but II is right
Correct Answer: B
Solution :
\[K=A{{e}^{-{{E}_{a}}/RT}}\] On increasing activation energy, K decreases & on increasing temperature K increases.You need to login to perform this action.
You will be redirected in
3 sec
