A)
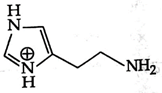
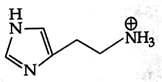
B)
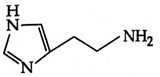
C)
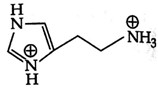
D)
Correct Answer: B
Solution :
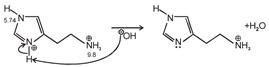 Isoelectronic point \[=\frac{5.74+9.8}{2}=7.77\] Histamine has two basic center which are Aliphatic amino group and imidazole ring. In blood, the aliphatic amino group (pKa = 9.4) will be exist whereas the second nitrogen of imidazole ring (pKa = 5.8) will not be protonated in blood which pH is 7.4. \[\text{pH=pKa+log}\frac{\text{ }\!\![\!\!\text{ Salt }\!\!]\!\!\text{ }}{\text{ }\!\![\!\!\text{ Acid }\!\!]\!\!\text{ }}\] \[\text{7}\text{.4=}\underset{\begin{smallmatrix} \downarrow \\ \text{It should be high for balancing} \end{smallmatrix}}{\mathop{\text{7}\text{.77+log}\frac{\text{ }\!\![\!\!\text{ Salt }\!\!]\!\!\text{ }}{\text{ }\!\![\!\!\text{ Acid }\!\!]\!\!\text{ }}}}\,\]
Isoelectronic point \[=\frac{5.74+9.8}{2}=7.77\] Histamine has two basic center which are Aliphatic amino group and imidazole ring. In blood, the aliphatic amino group (pKa = 9.4) will be exist whereas the second nitrogen of imidazole ring (pKa = 5.8) will not be protonated in blood which pH is 7.4. \[\text{pH=pKa+log}\frac{\text{ }\!\![\!\!\text{ Salt }\!\!]\!\!\text{ }}{\text{ }\!\![\!\!\text{ Acid }\!\!]\!\!\text{ }}\] \[\text{7}\text{.4=}\underset{\begin{smallmatrix} \downarrow \\ \text{It should be high for balancing} \end{smallmatrix}}{\mathop{\text{7}\text{.77+log}\frac{\text{ }\!\![\!\!\text{ Salt }\!\!]\!\!\text{ }}{\text{ }\!\![\!\!\text{ Acid }\!\!]\!\!\text{ }}}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
