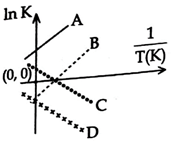
A) C and D
B) A and D
C) A and B
D) B and C
Correct Answer: C
Solution :
\[\text{InK=InA-}\frac{\Delta \text{H}}{\text{RT}}\text{,}\] For exothermic Reaction \[\Delta H=-ve\] slope\[=\left( -\frac{\Delta H}{R} \right)=+ve\].You need to login to perform this action.
You will be redirected in
3 sec
