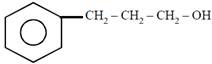-
question_answer1) If m and e are the mass and charge of the revolving electron in the orbit of radius r for hydrogen atom, the total energy of the revolving electron will be:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[\frac{1}{2}\frac{{{e}^{2}}}{r}\]
done
clear
B)
\[-\frac{{{e}^{2}}}{r}\]
done
clear
C)
\[m{{e}^{2}}\]
done
clear
D)
\[1\,{{e}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer2) The de-Broglie wavelength of a particle of mass 6.63 g moving with a velocity of 100 \[m{{s}^{-1}}\] is:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[{{10}^{-33}}m\]
done
clear
B)
\[{{10}^{-35}}m\]
done
clear
C)
\[{{10}^{-31}}m\]
done
clear
D)
\[{{10}^{-25}}m\]
done
clear
View Answer play_arrow
-
question_answer3) What happens when an inert gas is added to an equilibrium keeping volume unchanged?
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
More product will form
done
clear
B)
Less product will form
done
clear
C)
More reactant will form
done
clear
D)
Equilibrium will remain unchanged
done
clear
View Answer play_arrow
-
question_answer4) The amount of \[BaS{{O}_{4}}\]formed upon mixing 100 mL of 20.8% \[{{H}_{2}}S{{O}_{4}}\] solution with 50 mL of 9.8% \[{{H}_{2}}S{{O}_{4}}\]solution with 50 mL of 9.8% \[{{H}_{2}}S{{O}_{4}}\] solution will be: (Ba = 137, Cl = 35.5, S = 32, H = 1 and O = 16)
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
23.3 g
done
clear
B)
11.65 g
done
clear
C)
30.6 g
done
clear
D)
33.2 g
done
clear
View Answer play_arrow
-
question_answer5) The rate coefficient (k) for a particular reactions is \[1.3\times {{10}^{-4}}{{M}^{-1}}\]\[{{s}^{-1}}\] at \[100{}^\circ C\], and \[1.3\times {{10}^{-3}}\] at \[150{}^\circ C\]. What is the energy of activation (EA) (in kJ) for this reaction? (R = molar gas constant\[=8.31J{{K}^{-1}}kJ\])
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
16
done
clear
B)
60
done
clear
C)
99
done
clear
D)
132
done
clear
View Answer play_arrow
-
question_answer6) How many electrons would be required to deposit 6.35 g of copper at the cathode during the electrolysis of an aqueous solution of copper sulphate? (Atomic mass of copper \[=63.5u,{{N}_{A}}=\] Avogadro's constant):
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[\frac{{{N}_{A}}}{20}\]
done
clear
B)
\[\frac{{{N}_{A}}}{10}\]
done
clear
C)
\[\frac{{{N}_{A}}}{5}\]
done
clear
D)
\[\frac{{{N}_{A}}}{2}\]
done
clear
View Answer play_arrow
-
question_answer7) The \[\left( S{}^\circ \right)\] of the following substances are: \[C{{H}_{4}}(g)186.2J{{K}^{-1}}mo{{l}^{-1}}\] \[{{O}_{2}}(g)205.2J{{K}^{-1}}mo{{l}^{-1}}\] \[{{H}_{2}}O(g)69.9J{{K}^{-1}}mo{{l}^{-1}}\] The entropy change \[(\Delta {{S}^{o}})\]for the reaction : \[C{{H}_{4}}(g)+2{{O}_{2}}(g)\to C{{O}_{2}}(g)+2{{H}_{2}}O(l)\]is:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[-312.5J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
B)
\[-242.8J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
C)
\[-108.1J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
D)
\[-37.6J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer8) The conjugate base of hydrazoic acid is:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[{{N}^{-3}}\]
done
clear
B)
\[N_{3}^{-}\]
done
clear
C)
\[N_{2}^{-}\]
done
clear
D)
\[HN_{3}^{-}\]
done
clear
View Answer play_arrow
-
question_answer9) In a monoclinic unit cell, the relation of sides and angles are respectively:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[a=b\ne c\]and \[\alpha =\beta =\gamma ={{90}^{o}}\]
done
clear
B)
\[a\ne b\ne c\]and \[\alpha =\beta =\gamma ={{90}^{o}}\]
done
clear
C)
\[a\ne b\ne c\]and \[\beta =\gamma ={{90}^{o}}\ne \alpha \]
done
clear
D)
\[a\ne b\ne c\]and \[\alpha \ne \beta \ne \gamma \ne {{90}^{o}}\]
done
clear
View Answer play_arrow
-
question_answer10) The standard enthalpy of formation \[({{\Delta }_{f}}{{H}^{o}}_{298})\]for methane, \[C{{H}_{4}}\]is\[-74.9kJ\,mo{{l}^{-1}}.\]In order to calculate the average energy given out in the formation of a C ? H bond from this it is necessary to know which one of the following?
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
The dissociation energy of the hydrogen molecule, \[{{H}_{2}}\].
done
clear
B)
The first four ionisation energies of carbon.
done
clear
C)
The dissociation energy of \[{{H}_{2}}\]and enthalpy and sublimation of carbon (graphite).
done
clear
D)
The first four ionisation energies of carbon and electron affinity of hydrogen.
done
clear
View Answer play_arrow
-
question_answer11) Which of the following xenon-oxo compounds may not be obtained by hydrolysis of xenon fluorides?
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[Xe{{O}_{2}}{{F}_{2}}\]
done
clear
B)
\[XeO{{F}_{4}}\]
done
clear
C)
\[Xe{{O}_{3}}\]
done
clear
D)
\[Xe{{O}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer12) Excited hydrogen atom emits light in the ultraviolet region at \[2.47\times {{10}^{15}}Hz.\]With this frequency, the energy of a single photon is: \[(h=6.63\times {{10}^{-34}}Js)\]
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[8.041\times {{10}^{-40}}J\]
done
clear
B)
\[2.680\times {{10}^{-19}}J\]
done
clear
C)
\[1.640\times {{10}^{-18}}J\]
done
clear
D)
\[6.111\times {{10}^{-17}}J\]
done
clear
View Answer play_arrow
-
question_answer13) Which one of the following exhibits the large number of oxidation states?
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
Ti (22)
done
clear
B)
V (23)
done
clear
C)
Cr (24)
done
clear
D)
Mn (25)
done
clear
View Answer play_arrow
-
question_answer14) Copper becomes green when exposed to moist air for a long period. This is due to:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
the formation of a layer of cupric oxide on the surface of copper.
done
clear
B)
the formation of a layer of basic carbonate of copper on the surface of copper.
done
clear
C)
the formation of a layer of cupric hydroxide on the surface of copper.
done
clear
D)
the formation of basic copper sulphate layer on the surface of the metal.
done
clear
View Answer play_arrow
-
question_answer15) Among the following species the one which causes the highest \[CFSE,{{\Delta }_{0}}\]as a ligand is:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[C{{N}^{-}}\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[{{F}^{-}}\]
done
clear
D)
CO
done
clear
View Answer play_arrow
-
question_answer16) Similarity in chemical properties of the atoms of elements in a group of the Periodic table is most closely related to:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
atomic numbers
done
clear
B)
atomic masses
done
clear
C)
number of principal energy levels
done
clear
D)
number of valence electrons
done
clear
View Answer play_arrow
-
question_answer17) Which of the following arrangements represents the increasing order (smallest to largest) of ionic radii of the given species \[{{O}^{2-}},{{S}^{2-}},{{N}^{3-}},{{p}^{3-?}}\]
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[{{O}^{2-}}<{{N}^{3-}}<{{S}^{2-}}<{{p}^{3-}}\]
done
clear
B)
\[{{O}^{2-}}<{{p}^{3-}}<{{N}^{3-}}<{{S}^{2-}}\]
done
clear
C)
\[{{N}^{3}}<{{O}^{2-}}<{{p}^{3-}}<{{S}^{2-}}\]
done
clear
D)
\[{{N}^{3-}}<{{S}^{2-}}<{{O}^{2-}}<{{p}^{3-}}\]
done
clear
View Answer play_arrow
-
question_answer18) Global warming is due to increase of:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
methane and nitrous oxide in atmosphere
done
clear
B)
methane and \[C{{O}_{2}}\] in atmosphere
done
clear
C)
methane and \[{{O}_{3}}\] in atmosphere
done
clear
D)
methane and CO in atmosphere
done
clear
View Answer play_arrow
-
question_answer19) Hydrogen peroxide acts both as an oxidising and as a reducing agent depending upon the nature of the reacting species. In which of the following cases \[{{H}_{2}}{{O}_{2}}\]acts as a reducing agent in acid medium?
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[MnO_{4}^{-}\]
done
clear
B)
\[C{{r}_{2}}O_{7}^{2-}\]
done
clear
C)
\[SO_{3}^{2-}\]
done
clear
D)
KI
done
clear
View Answer play_arrow
-
question_answer20) Which one of the following complexes will most likely absorb visible light? (At nos. Sc = 21, Ti = 22, V = 23, Zn = 30)
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[{{[Sc{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
done
clear
B)
\[{{[Ti{{(N{{H}_{3}})}_{6}}]}^{4+}}\]
done
clear
C)
\[{{[V{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
D)
\[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{2+}}\]
done
clear
View Answer play_arrow
-
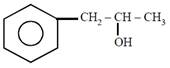
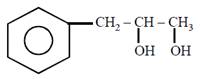
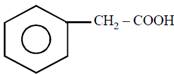
question_answer21)
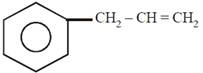 on mercuration-demercuration produces the major product:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
on mercuration-demercuration produces the major product:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer22) In the Victor-Meyer's test, the colour given by \[1{}^\circ ,\text{ }2{}^\circ \text{ }and\text{ }3{}^\circ \] alcohols are respectively:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
Red, colourless, blue
done
clear
B)
Red, blue, colourless
done
clear
C)
Colourless, red, blue
done
clear
D)
Red, blue, violet
done
clear
View Answer play_arrow
-
question_answer23) Conversion of benzene diazonium chloride to chlorobenzene is an example of which of the following reactions?
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
Claisen
done
clear
B)
Friedel-craft
done
clear
C)
Sandmeyer
done
clear
D)
Wurtz
done
clear
View Answer play_arrow
-
question_answer24) In the presence of peroxide, HCl and HI do not give anti- Markownikoff's addition of alkenes because:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
One of the steps is endothermic in HCl and HI
done
clear
B)
Both HCl and HI are strong acids
done
clear
C)
HCl is oxidizing and the HI is reducing
done
clear
D)
All the steps are exothermic is HCl and HI
done
clear
View Answer play_arrow
-
question_answer25) The major product obtained in the photo catalyzed bromination of 2--methylbutane is:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
1-bromo-2-methylbutane
done
clear
B)
1-bromo-3-methylbutane
done
clear
C)
2-bromo-3-methylbutane
done
clear
D)
2-bromo-2-methylbutane
done
clear
View Answer play_arrow
-
question_answer26) Which of the following molecules has two sigma (s) and two \[{{p}_{i}}(\pi )\]bonds?
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
B)
\[{{N}_{2}}{{F}_{2}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{2}}C{{l}_{2}}\]
done
clear
D)
HCN
done
clear
View Answer play_arrow
-
question_answer27) Which one of the following acids does not exhibit optical isomerism?
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
Lactic acid
done
clear
B)
Tartaric acid
done
clear
C)
Maleic acid
done
clear
D)
\[\text{ }\!\!\alpha\!\!\text{ -}\]amino acids
done
clear
View Answer play_arrow
-
question_answer28) Aminoglycosides are usually used as:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
antibiotic
done
clear
B)
analgesic
done
clear
C)
hypnotic
done
clear
D)
antifertility
done
clear
View Answer play_arrow
-
question_answer29) Which of the following will not show mutarotation?
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
Maltose
done
clear
B)
Lactose
done
clear
C)
Glucose
done
clear
D)
Sucrose
done
clear
View Answer play_arrow
-
question_answer30) Phthalic acid reacts with resorcinol in the presence of concentrated \[{{H}_{2}}S{{O}_{4}}\]to give:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
A)
Phenolphthalein
done
clear
B)
Alizarin
done
clear
C)
Coumarin
done
clear
D)
Fluorescein
done
clear
View Answer play_arrow
 on mercuration-demercuration produces the major product:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )
on mercuration-demercuration produces the major product:
[JEE Main Online Paper ( Held On 12 Apirl 2014 )