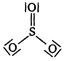
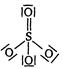
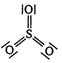
question_answer 1) In \[PO_{4}^{3-}\] ion, the formal charge on each oxygen atom and \[P-O\] bond order respectively are: [AIPMT 1998]
A)
\[-\text{ }0.75,\text{ }0.6\]
done
clear
B)
\[-\text{ }0.75,\text{ }1.0\]
done
clear
C)
\[-\text{ }0.75,\text{ }1.25\]
done
clear
D)
\[-\text{ }3,\text{ }1.25\]
done
clear
View Answer play_arrow
question_answer 2) The number of anti-bonding electrons in \[O_{2}^{2-}\] molecular ion on the basis of molecular orbital theory is (Atomic no. of O is 8): [AIPMT 1998]
A)
5
done
clear
B)
2
done
clear
C)
4
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 3) The most suitable method of the separation of a mixture of ortho and para-nitrophenol mixed in the ratio of 1 : 1 is: [AIPMT 1999]
A)
distillation
done
clear
B)
crystallization
done
clear
C)
vaporization
done
clear
D)
colour spectrum
done
clear
View Answer play_arrow
question_answer 4) The type of hybridisation of boron in diborane is: [AIPMT 1999]
A)
sp-hybridisation
done
clear
B)
\[s{{p}^{2}}\]-hybridisation
done
clear
C)
\[s{{p}^{3}}\]-hybridisation
done
clear
D)
\[s{{p}^{3}}{{d}^{2}}\]-hybridisation
done
clear
View Answer play_arrow
question_answer 5) Which one of the following is planar?
A)
\[Xe{{F}_{4}}\]
done
clear
B)
\[Xe{{O}_{4}}\]
done
clear
C)
\[Xe{{O}_{3}}F\]
done
clear
D)
\[Xe{{O}_{3}}{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 6) Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [AIPMT 2000]
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
HCl
done
clear
D)
HF
done
clear
View Answer play_arrow
question_answer 7) Among the following the electron deficient compound is: [AIPMT 2000]
A)
\[BC{{l}_{3}}\]
done
clear
B)
\[CC{{l}_{4}}\]
done
clear
C)
\[PC{{l}_{5}}\]
done
clear
D)
\[BeC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 8) The relationship between the dissociation energy of \[{{N}_{2}}\] and \[N_{2}^{+}\] is: [AIPMT 2000]
A)
dissociation energy of \[N_{2}^{+}>\] dissociation energy of \[N_{2}^{+}\]
done
clear
B)
dissociation energy of \[{{N}_{2}}=\] dissociation energy of \[N_{2}^{+}\]
done
clear
C)
dissociation energy of \[{{N}_{2}}>\] dissociation energy of \[N_{2}^{+}\]
done
clear
D)
dissociation energy of \[{{N}_{2}}\] can either be lower or higher than the dissociation energy of \[N_{2}^{+}\]
done
clear
View Answer play_arrow
question_answer 9) Which one of the following is not paramagnetic? [AIPMT 2000]
A)
NO
done
clear
B)
\[N_{2}^{+}\]
done
clear
C)
CO
done
clear
D)
\[O_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 10) Which of the following two are isostructural? [AIPMT 2001]
A)
\[Xe{{F}_{2}},\,IF_{2}^{-}\]
done
clear
B)
\[N{{H}_{3}},\,B{{F}_{3}}\]
done
clear
C)
\[CO_{3}^{2-},\,SO_{3}^{2-}\]
done
clear
D)
\[PC{{l}_{5}},\,IC{{l}_{5}}\]
done
clear
View Answer play_arrow
question_answer 11) In which of the following, bond angle is maximum? [AIPMT 2001]
A)
\[N{{H}_{3}}\]
done
clear
B)
\[NH_{4}^{+}\]
done
clear
C)
\[PC{{I}_{3}}\]
done
clear
D)
\[SC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 12) In \[X-H.......Y,\,\,X\] and Y both are electronegative elements: [AIPMT 2001]
A)
electron density on X will increase and on H will decrease
done
clear
B)
in both electron density will increase
done
clear
C)
in both electron density will decrease
done
clear
D)
on X electron density will decrease and at H increase
done
clear
View Answer play_arrow
question_answer 13) Main axis of a diatomic molecule is z, molecular orbital \[{{p}_{x}}\] and \[{{p}_{y}}\] overlaps to form, which of the orbital? [AIPMT 2001]
A)
\[\pi -\]molecular orbital
done
clear
B)
\[\sigma -\]molecular orbital
done
clear
C)
\[\delta -\]molecular orbital
done
clear
D)
No bond will form
done
clear
View Answer play_arrow
question_answer 14) In \[NO_{3}^{-}\] ion number of bond pair and lone pair of electron on nitrogen atom are: [AIPMT 2002]
A)
2, 2
done
clear
B)
3, 1
done
clear
C)
1, 3
done
clear
D)
4, 0
done
clear
View Answer play_arrow
question_answer 15) Among the following, the pair in which the two species are not isostructural, is: [AIPMT (S) 2004]
A)
\[Si{{F}_{4}}\] and \[S{{F}_{4}}\]
done
clear
B)
\[IO_{3}^{-}\] and \[Xe{{O}_{3}}\]
done
clear
C)
\[B{{H}_{4}}\] and \[NH_{4}^{+}\]
done
clear
D)
\[PF_{6}^{-}\] and \[SF_{6}^{{}}\]
done
clear
View Answer play_arrow
question_answer 16) In a regular octahedral molecule, \[M{{X}_{6}}\] the number of \[X-M-X\] bonds at 180° is: [AIPMT (S) 2004]
A)
three
done
clear
B)
two
done
clear
C)
six
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 17) \[{{H}_{2}}O\] is dipolar, whereas \[Be{{F}_{2}}\] is not. It is because: [AIPMT (S) 2004]
A)
the electro negativity of F is greater than that of O
done
clear
B)
\[{{H}_{2}}O\] involves hydrogen bonding whereas\[Be{{F}_{2}}\] is a discrete molecule
done
clear
C)
\[{{H}_{2}}O\]is linear and \[Be{{F}_{2}}\] is angular
done
clear
D)
\[{{H}_{2}}O\] is angular and \[Be{{F}_{2}}\] is linear
done
clear
View Answer play_arrow
question_answer 18) In \[Br{{F}_{3}}\] molecule, the lone pairs occupy equatorial positions to minimize: [AIPMT (S) 2004]
A)
lone pair-bond pair repulsion only
done
clear
B)
bond pair-bond pair repulsion only
done
clear
C)
lone pair-lone pair repulsion and lone pair-bond pair repulsion
done
clear
D)
lone pair-lone pair repulsion only
done
clear
View Answer play_arrow
question_answer 19) The correct order in which the \[O-O\] bond length increases in the following is: [AIPMT (S) 2005]
A)
\[{{H}_{2}}{{O}_{2}}<{{O}_{2}}<{{O}_{3}}\]
done
clear
B)
\[{{O}_{3}}<{{H}_{2}}{{O}_{2}}<{{O}_{2}}\]
done
clear
C)
\[{{O}_{2}}<{{O}_{3}}<{{H}_{2}}{{O}_{2~}}\]
done
clear
D)
\[{{O}_{2}}<{{H}_{2}}{{O}_{2}}<{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 20) Which of the following molecules has trigonal planar geometry? [AIPMT (S) 2005]
A)
\[I{{F}_{3}}~~\]
done
clear
B)
\[PC{{l}_{3}}\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[B{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 21) The correct sequence of increasing covalent character is represented by: [AIPMT (S) 2005]
A)
\[LiCl<NaCl<BeC{{l}_{2}}\]
done
clear
B)
\[~BeC{{l}_{2}}<NaCl<LiCl\]
done
clear
C)
\[~NaCl<LiCl<BeC{{l}_{2}}\]
done
clear
D)
\[BeC{{l}_{2}}<LiCl<NaCl\]
done
clear
View Answer play_arrow
question_answer 22) Which of the following is the electron deficient molecule? [AIPMT (S) 2005]
A)
\[{{B}_{2}}{{H}_{6}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[P{{H}_{3}}\]
done
clear
D)
\[Si{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 23) The surface tension of which of the following liquid is maximum? [AIPMT (S) 2005]
A)
\[{{H}_{2}}O\]
done
clear
B)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
C)
\[C{{H}_{3}}OH\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 24) Which of the following would have a permanent dipole moment? [AIPMT (S) 2005]
A)
\[B{{F}_{3}}\]
done
clear
B)
\[Si{{F}_{4}}\]
done
clear
C)
\[S{{F}_{4}}\]
done
clear
D)
\[Xe{{F}_{4}}\]
done
clear
View Answer play_arrow
question_answer 25) Which one of the following oxides is expected to exhibit paramagnetic behaviour? [AIPMT (S) 2005]
A)
\[C{{O}_{2}}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[Cl{{O}_{2}}\]
done
clear
D)
\[Si{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 26) In which of the following molecules are all the bonds not equal? [AIPMT (S) 2006]
A)
\[Cl{{F}_{3}}\]
done
clear
B)
\[B{{F}_{3}}\]
done
clear
C)
\[Al{{F}_{3}}\]
done
clear
D)
\[N{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 27) The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of \[N{{H}_{3}}\] (1.5 D) is larger than that of \[N{{F}_{3}}\] (0.2 D). This is because: [AIPMT (S) 2006]
A)
in \[N{{H}_{3}}\] as well as in \[N{{F}_{3}}\] the atomic dipole and bond dipole are in the same direction
done
clear
B)
in \[N{{H}_{3}}\] the atomic dipole and bond dipole are in the same direction whereas in \[N{{F}_{3}}\] these are in opposite directions
done
clear
C)
in \[N{{H}_{3}}\] as well as \[N{{F}_{3}}\] the atomic dipole and bond dipole are in opposite directions
done
clear
D)
in \[N{{H}_{3}}\] the atomic dipole and bond dipole are in the opposite directions whereas in \[N{{F}_{3}}\]these are in the same directions
done
clear
View Answer play_arrow
question_answer 28) The correct order regarding the electronegativity of hybrid orbitals of carbon is: [AIPMT(S) 2006]
A)
\[sp>s{{p}^{2}}<s{{p}^{3}}\]
done
clear
B)
\[sp>s{{p}^{2}}>s{{p}^{3}}\]
done
clear
C)
\[sp<s{{p}^{2}}>s{{p}^{3}}\]
done
clear
D)
\[sp<s{{p}^{2}}<s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 29) Which of the following species has a linear shape?
A)
\[NO_{2}^{-}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[NO_{2}^{+}\]
done
clear
D)
\[{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 30) Which of the following is not isostructural with\[SiC{{l}_{4}}\]? [AIPMT (S) 2006]
A)
\[SC{{l}_{4}}\]
done
clear
B)
\[SO_{4}^{2-}\]
done
clear
C)
\[PO_{4}^{3-}\]
done
clear
D)
\[NH_{4}^{+}\]
done
clear
View Answer play_arrow
question_answer 31) The correct order of \[C-O\] bond length among \[CO,\,\,CO_{3}^{2-},\,C{{O}_{2}}\] is: [AIPMT (S) 2007]
A)
\[C{{O}_{2}}<CO_{3}^{2-}<CO\]
done
clear
B)
\[CO<CO_{3}^{2-}<C{{O}_{2}}\]
done
clear
C)
\[CO_{3}^{2-}<C{{O}_{2}}<CO~\]
done
clear
D)
\[CO<C{{O}_{2}}<CO_{3}^{2-}\]
done
clear
View Answer play_arrow
question_answer 32) In which of the following pairs, the two species are iso-structural? [AIPMT (S) 2007]
A)
\[S{{F}_{4}}\] and \[Xe{{F}_{4}}\]
done
clear
B)
\[SO_{3}^{2-}\] and \[NO_{3}^{-}\]
done
clear
C)
\[B{{F}_{3}}\] and \[N{{F}_{3}}\]
done
clear
D)
\[BrO_{3}^{-}\] and \[Xe{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 33) The angular shape of ozone molecule \[({{O}_{3}})\] consists of [AIPMT (S) 2008]
A)
1 sigma and 2 pi bonds
done
clear
B)
2 sigma and 2 pi bonds
done
clear
C)
1 sigma and 1 pi bonds
done
clear
D)
2 sigma and 1 pi bonds
done
clear
View Answer play_arrow
question_answer 34) The correct order of increasing bond angles in the following triatomic species is [AIPMT (S) 2008]
A)
\[NO_{2}^{-}<NO_{2}^{+}<N{{O}_{2}}\]
done
clear
B)
\[NO_{2}^{-}<N{{O}_{2}}<NO_{2}^{+}\]
done
clear
C)
\[NO_{2}^{+}<N{{O}_{2}}<NO_{2}^{-}\]
done
clear
D)
\[NO_{2}^{+}<NO_{2}^{-}<N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 35) Four diatomic species are listed below in different sequences. Which of these presents the correct order of their increasing bond order? [AIPMT (S) 2008]
A)
\[O_{2}^{-}<NO<C_{2}^{2-}<He_{2}^{+}\]
done
clear
B)
\[NO<C_{2}^{2-}<O_{2}^{-}He_{2}^{+}\]
done
clear
C)
\[C_{2}^{2-}<He_{2}^{+}<NO<O_{2}^{-}\]
done
clear
D)
\[He_{2}^{+}<O_{2}^{-}<NO<C_{2}^{2-}\]
done
clear
View Answer play_arrow
question_answer 36) In the hydrocarbon [AIPMT (S) 2008] \[\underset{6}{\mathop{C{{H}_{3}}}}\,-\underset{5}{\mathop{CH}}\,=\underset{4}{\mathop{CH}}\,-\underset{3}{\mathop{C{{H}_{2}}}}\,-\underset{2}{\mathop{C}}\,\equiv \underset{1}{\mathop{CH}}\,\] The state of hybridisation of carbons 1, 3 and 5 are in the following sequence
A)
\[~s{{p}^{2}},sp,\text{ }s{{p}^{3}}\]
done
clear
B)
\[sp,\text{ }s{{p}^{3}},\text{ }s{{p}^{2}}\]
done
clear
C)
\[sp,\text{ }s{{p}^{2}},\text{ }s{{p}^{3}}\]
done
clear
D)
\[s{{p}^{3}},s{{p}^{2}},\text{ }sp\]
done
clear
View Answer play_arrow
question_answer 37) According to MO theory which of the following lists ranks the nitrogen species in tends of increasing bond order? [AIPMT (S) 2009]
A)
\[\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{H} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{H} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,=\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{H} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{H} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,+\text{H}-\xrightarrow[{}]{{}}\text{H}-\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{H} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{H} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,-\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{H} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{H} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,-\text{H}\]
done
clear
B)
\[\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
C)
\[\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
D)
\[\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
View Answer play_arrow
question_answer 38) The state of hybridization of \[\text{mo}{{\text{l}}^{\text{-1}}}\] and \[1.77\times {{10}^{-5}}\]is in the following sequence [AIPMT (S) 2009]
A)
\[sp,\text{ }s{{p}^{3}},\text{ }s{{p}^{2}}\] and \[s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{3}},\text{ }s{{p}^{2}},\text{ }s{{p}^{2}}\] and sp
done
clear
C)
\[sp,\text{ }s{{p}^{2}},\text{ }s{{p}^{2}}\] and \[s{{p}^{3}}\]
done
clear
D)
\[sp,s{{p}^{2}},\text{ }s{{p}^{3}}\] and \[s{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 39) In the case of alkali metals, the covalent character decreases in the order [AIPMT (S) 2009]
A)
\[MCl>Ml>MBr>MF\]
done
clear
B)
\[MF>MCl>MBr>Ml\]
done
clear
C)
\[MF>MCl>MI>MBr\]
done
clear
D)
\[Al>MBr>MCl>MF\]
done
clear
View Answer play_arrow
question_answer 40) In which of the following molecules/ions \[B{{F}_{3}},\,NO_{2}^{-},\,NH_{2}^{-}\] and \[{{H}_{2}}O\], the central atom is sp2 hybridised? [AIPMT (S) 2009]
A)
\[NO_{2}^{-}\] and\[NH_{2}^{-}\]
done
clear
B)
\[NH_{2}^{-}\] and \[{{H}_{2}}O\]
done
clear
C)
\[NO_{2}^{-}\] and \[{{H}_{2}}O\]
done
clear
D)
\[B{{F}_{2}}\] and \[NO_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 41) What is the dominant intermolecular force or bond that must be overcome in converting liquid \[C{{H}_{3}}OH\] to a gas? [AIPMT (S) 2009]
A)
Hydrogen bonding
done
clear
B)
Dipole-dipole interaction
done
clear
C)
Covalent bonds
done
clear
D)
London dispersion force
done
clear
View Answer play_arrow
question_answer 42) In which of the following pairs of molecules/ions, the central atoms have sp2 hybridization? [AIPMT (S) 2010]
A)
\[NO_{2}^{-}\] and \[N{{H}_{3}}\]
done
clear
B)
\[BF_{3}^{{}}\] and \[NH_{2}^{-}\]
done
clear
C)
\[NH_{2}^{-}\] and \[H_{2}^{{}}O\]
done
clear
D)
\[BF_{3}^{{}}\] and
done
clear
View Answer play_arrow
question_answer 43) Which one of the following species does not exist under normal conditions? [AIPMT (S) 2010]
A)
x\[Be_{2}^{+}\]
done
clear
B)
\[Be_{2}^{{}}\]
done
clear
C)
\[{{B}_{2}}\]
done
clear
D)
\[Li_{2}^{{}}\]
done
clear
View Answer play_arrow
question_answer 44) The correct order of increasing bond angles in the following species is [AIPMT (S) 2010]
A)
\[C{{l}_{2}}O<Cl{{O}_{2}}<ClO_{2}^{-}\]
done
clear
B)
\[C{{l}_{2}}O<C{{l}_{2}}O<ClO_{2}^{-}\]
done
clear
C)
\[C{{l}_{2}}O<ClO_{2}^{-}<ClO_{2}^{{}}\]
done
clear
D)
\[ClO_{2}^{-}<C{{l}_{2}}O<ClO_{2}^{{}}\]
done
clear
View Answer play_arrow
question_answer 45) In which one of the following species the central atom has the type of hybridization which is not the same as that present in the other three? [AIPMT (S) 2010]
A)
\[S{{F}_{4}}\]
done
clear
B)
\[I_{3}^{-}\]
done
clear
C)
\[SbCl_{5}^{2-}\]
done
clear
D)
\[PC{{l}_{5}}\]
done
clear
View Answer play_arrow
question_answer 46) In which of the following molecules the central atom does not have sp3 hybridisation? [AIPMT (M) 2010]
A)
\[C{{H}_{4}}\]
done
clear
B)
\[S{{F}_{4}}\]
done
clear
C)
\[BF_{4}^{-}\]
done
clear
D)
\[NH_{4}^{+}\]
done
clear
View Answer play_arrow
question_answer 47) Some of the properties of the two species, \[NO_{3}^{-}\]and \[{{H}_{3}}O_{{}}^{+}\] are described below. Which one of them is correct? [AIPMT (M) 2010]
A)
Dissimilar in hybridisation for the central atom with different structures
done
clear
B)
Isostructural with same hybridisation for the central atom
done
clear
C)
Isostructural with different hybridization for the central atom
done
clear
D)
Similar in hybridisation for the central atom with different structures
done
clear
View Answer play_arrow
question_answer 48) Considering the state of hybridisation of carbon atoms, find out the molecule among the following which is linear. [AIPMT (S) 2011]
A)
\[C{{H}_{3}}-C\equiv C-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{2}}=CH-C{{H}_{2}}-C\equiv CH\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-CH=CH-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 49) Which of the two ions from the list given below, have the geometry that is explained by the same hybridisation of orbitals, \[NO_{2}^{-},NO_{3}^{-},\]\[NH_{2}^{-},\]\[NH_{4}^{+},\]\[SC{{N}^{-}}?\] [AIPMT (S) 2011]
A)
\[NH_{4}^{-}\] and \[NO_{3}^{-}\]
done
clear
B)
\[SCN_{{}}^{+}\] and \[NO_{2}^{-}\]
done
clear
C)
\[NO_{2}^{-}\] and \[NH_{2}^{-}\]
done
clear
D)
\[NO_{2}^{-}\] and \[NH_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 50) Which of the following compounds has the lowest melting point? [AIPMT (S) 2011]
A)
\[CaB{{r}_{2}}\]
done
clear
B)
\[Ca{{l}_{2}}\]
done
clear
C)
\[Ca{{F}_{2}}\]
done
clear
D)
\[CaC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 51) Which of the following has the minimum bond length? [AIPMT (S) 2011]
A)
\[O_{2}^{-}\]
done
clear
B)
\[O_{2}^{2-}\]
done
clear
C)
\[O_{2}^{{}}\]
done
clear
D)
\[O_{2}^{+}\]
done
clear
View Answer play_arrow
question_answer 52) The correct order of increasing bond length of \[C-H,C-O,C-C\] and \[C=C\] is [AIPMT (S) 2011]
A)
\[C-C<C=C<C-O<C-H\]
done
clear
B)
\[C-O<C-H<C-C<C=C\]
done
clear
C)
\[C-H<C-O<C-C<C=C\]
done
clear
D)
\[C-H<C=C<C-O<C-C\]
done
clear
View Answer play_arrow
question_answer 53) Which of the following structures is the most preferred and hence of lowest energy for\[S{{O}_{3}}\]? [AIPMT (M) 2011]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 54) The pairs of species of oxygen and their magnetic behaviors are noted below. Which of the following presents the correct description? [AIPMT (M) 2011]
A)
\[O_{2}^{-},O_{2}^{2-}-\]Both diamagnetic
done
clear
B)
\[{{O}^{+}},\,O_{2}^{2-}-\]Both paramagnetic
done
clear
C)
\[O_{2}^{+},\,{{O}_{2}}-\] Both paramagnetic
done
clear
D)
\[O_{{}}^{{}},O_{2}^{2-}-\]Both paramagnetic
done
clear
View Answer play_arrow
question_answer 55) Which one of the following pairs is isostructural (i.e., having the same shape and hybridization)? [AIPMT (S) 2012]
A)
\[[BC{{l}_{3}}\,\text{and}\,BrC{{l}_{3}}]\]
done
clear
B)
\[[N{{H}_{3}}\,\text{and}\,NO_{3}^{-}]\]
done
clear
C)
\[[N{{F}_{3}}\,\text{and}\,B{{F}_{3}}]\]
done
clear
D)
\[[BF_{4}^{-}\,\text{and}\,NH_{4}^{+}]\]
done
clear
View Answer play_arrow
question_answer 56) Bond order of 1.5 is shown by [AIPMT (S) 2012]
A)
\[O_{2}^{+}\]
done
clear
B)
\[O_{2}^{-}\]
done
clear
C)
\[O_{2}^{2-}\]
done
clear
D)
\[O_{2}^{{}}\]
done
clear
View Answer play_arrow
question_answer 57) Which of the following species contains three bond pairs and one lone pair around the central atom? [AIPMT (S) 2012]
A)
\[{{H}_{2}}O\]
done
clear
B)
\[B{{F}_{3}}\]
done
clear
C)
\[NH_{2}^{-}\]
done
clear
D)
\[PC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 58) The pair of species with the same bond order is [AIPMT (S) 2012]
A)
\[O_{2}^{2-},{{B}_{2}}\]
done
clear
B)
\[O_{2}^{+},N{{O}^{+}}\]
done
clear
C)
\[NO,CO\]
done
clear
D)
\[{{N}_{2}},{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 59) During change of \[{{O}_{2}}\] to \[O_{2}^{-}\] ion, the electron adds on which one of the following orbitals? [AIPMT (M) 2012]
A)
\[\overset{\text{*}}{\mathop{\text{ }\!\!\pi\!\!\text{ }}}\,\]orbital
done
clear
B)
\[\text{ }\!\!\pi\!\!\text{ }\]orbital
done
clear
C)
\[\overset{\text{*}}{\mathop{\sigma }}\,\]orbital
done
clear
D)
\[\overset{{}}{\mathop{\sigma }}\,\]
done
clear
View Answer play_arrow
question_answer 60)
Four diatomic species are listed below. Identify the correct order in which the bond order is increasing in them. [AIPMT (M) 2012]
A)
\[NO<O_{2}^{-}<C_{2}^{2-}<He_{2}^{+}\]
done
clear
B)
\[O_{2}^{-}<NO<C_{2}^{2-}<He_{2}^{+}\]
done
clear
C)
\[C_{2}^{2-}<He_{2}^{+}<O_{2}^{-}<NO\]
done
clear
D)
\[He_{2}^{+}<O_{2}^{-}<NO<C_{2}^{2-}\]
done
clear
View Answer play_arrow
question_answer 61) Dipole-induced dipole interactions are present in which of the following pairs? [NEET 2013]
A)
\[{{H}_{2}}O\] and alcohol
done
clear
B)
\[C{{l}_{2}}\] and \[CC{{l}_{4}}\]
done
clear
C)
\[HCl\] and \[He\] atoms
done
clear
D)
\[Si{{F}_{4}}\] and \[He\]atoms
done
clear
View Answer play_arrow
question_answer 62) Which of the following is paramagnetic? [NEET 2013]
A)
\[CO\]
done
clear
B)
\[O_{2}^{-}\]
done
clear
C)
\[C{{N}^{-}}\]
done
clear
D)
\[N{{O}^{+}}\]
done
clear
View Answer play_arrow
question_answer 63) Identify the correct order of solubility in aqueous medium. [NEET 2013]
A)
\[CuS\,>\,Zn\,>\,N{{a}_{2}}S\]
done
clear
B)
\[ZnS\,>\,N{{a}_{2}}S\,\,>\,CuS\]
done
clear
C)
\[N{{a}_{2}}S\,\,>\,CuS\,>ZnS\]
done
clear
D)
\[N{{a}_{2}}S\,\,>\,ZnS\,>\,CuS\]
done
clear
View Answer play_arrow
question_answer 64) \[Xe{{F}_{2}}\] is structural with [NEET 2013]
A)
\[Te{{F}_{2}}\]
done
clear
B)
\[lCl_{2}^{-}\]
done
clear
C)
\[SbC{{l}_{3}}\]
done
clear
D)
\[BaC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 65) Which one of the following molecules contain no \[\text{ }\!\!\pi\!\!\text{ -}\]bond? [NEET 2013]
A)
\[C{{O}_{2}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 66) Which of the following is a polar molecule? [NEET 2013]
A)
\[B{{F}_{3}}\]
done
clear
B)
\[S{{F}_{4}}\]
done
clear
C)
\[Si{{F}_{4}}\]
done
clear
D)
\[Xe{{F}_{4}}\]
done
clear
View Answer play_arrow
question_answer 67)
The radical
A)
6 p-orbitals and 6 unpaired electrons
done
clear
B)
7 p-orbitals and 6 unpaired electrons
done
clear
C)
7 p-orbitals and 7 unpaired electrons
done
clear
D)
6 p-orbitals and 7 unpaired electrons
done
clear
View Answer play_arrow
question_answer 68) Which of the following molecules has the maximum dipole moment? [AIPMT 2014]
A)
\[C{{O}_{2}}\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[N{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 69) Which one of the following species has plane triangular shape? [AIPMT 2014]
A)
\[{{N}_{3}}\]
done
clear
B)
\[NO_{3}^{-}\]
done
clear
C)
\[N{{O}_{2}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 70) Which of the following species contains equal number of \[\sigma \] and \[\pi \]-bonds? [NEET 2015 ]
A)
\[HCO_{3}^{-}\]
done
clear
B)
\[Xe{{O}_{4}}\]
done
clear
C)
\[{{(CN)}_{2}}\]
done
clear
D)
\[C{{H}_{2}}{{(CN)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 71) Which of the following pairs of ions are isoelectronic and isostructural? [NEET 2015]
A)
\[CO_{3}^{2-},SO_{3}^{2-}\]
done
clear
B)
\[ClO_{3}^{-},\,CO_{3}^{2-}\]
done
clear
C)
\[SO_{3}^{2-},NO_{3}^{-}\]
done
clear
D)
\[ClO_{3}^{-},SO_{3}^{2-}\]
done
clear
View Answer play_arrow
question_answer 72) Which of the following options represents the correct bond order? [NEET 2015 ]
A)
\[O_{2}^{-}>{{O}_{2}}>O_{2}^{+}\]
done
clear
B)
\[O_{2}^{-}<{{O}_{2}}<O_{2}^{+}\]
done
clear
C)
\[O_{2}^{-}>{{O}_{2}}<O_{2}^{+}\]
done
clear
D)
\[O_{2}^{-}<{{O}_{2}}>O_{2}^{+}\]
done
clear
View Answer play_arrow
question_answer 73) Maximum bond angle at nitrogen is present in which of the following? [NEET 2015 ]
A)
\[N{{O}_{2}}\]
done
clear
B)
\[NO_{2}^{-}\]
done
clear
C)
\[NO_{2}^{+}\]
done
clear
D)
\[NO_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 74) The correct bond order in the following species is [NEET 2015 ]
A)
\[O_{2}^{2+}>O_{2}^{+}>O_{2}^{-}\]
done
clear
B)
\[O_{2}^{2+}<O_{2}^{-}<O_{2}^{+}\]
done
clear
C)
\[O_{2}^{+}>O_{2}^{-}<O_{2}^{2+}\]
done
clear
D)
\[O_{2}^{-}<O_{2}^{+}>O_{2}^{2+}\]
done
clear
View Answer play_arrow
question_answer 75)
The total number of \[\pi -\]bond electrons in the following structure is [NEET 2015]
A)
4
done
clear
B)
8
done
clear
C)
12
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 76) .In which of the following pairs, both the species are not isostructural? [NEET 2015 (Re)]
A)
\[SiC{{l}_{4}},PCl_{4}^{+}\]
done
clear
B)
Diamond, carbide
done
clear
C)
\[N{{H}_{3}},P{{H}_{3}}\]
done
clear
D)
\[Xe{{F}_{4}},Xe{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 77) Decreasing order of stability of \[{{O}_{2}},O_{2}^{-},O_{2}^{+}\] and \[O_{2}^{2-}\]is [NEET 2015 (Re)]
A)
\[O_{2}^{+}>{{O}_{2}}>O_{2}^{-}>O_{2}^{2-}\]
done
clear
B)
\[O_{2}^{2-}>O_{2}^{-}>{{O}_{2}}>O_{2}^{+}\]
done
clear
C)
\[{{O}_{2}}>O_{2}^{+}>O_{2}^{2-}>O_{2}^{-}\]
done
clear
D)
\[O_{2}^{-}>O_{2}^{2-}>O_{2}^{+}>{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 78) Consider the molecules \[C{{H}_{4}},N{{H}_{3}}\] and \[{{H}_{2}}O\]. Which of the given statements is false? [NEET - 2016]
A)
The \[HCH\] bond angle in \[C{{H}_{4}},\] the \[HN\]bond angle in \[N{{H}_{3}},\] and the \[HOH\] bond angle in \[{{H}_{2}}O\] are all greater than \[{{90}^{o}}\]
done
clear
B)
The \[HOH\] bond angle in \[{{H}_{2}}O\] is larger than the \[HCH\] bond angle in \[C{{H}_{4}}\]
done
clear
C)
The \[HOH\] bond angle in \[{{H}_{2}}O\] is smaller than the x\[HNH\] bond angle in \[N{{H}_{3}}\]
done
clear
D)
The \[HCH\] bond angle in \[C{{H}_{4}}\] is larger than the \[HNH\] bond angle in \[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 79) Predict the correct order among the following: [NEET - 2016]
A)
lone pair- lone pair > lone pair - bond pair bond pair - bond pair
done
clear
B)
lone pair - lone pair > bond pair - bond pair lone pair - bond pair
done
clear
C)
bond pair - bond pair > lone pair - bond pair lone pair - lone pair
done
clear
D)
lone pair - bond pair > bond pair - bond pair lone pair - lone pair
done
clear
View Answer play_arrow
question_answer 80)
Match the compounds given in column I with the hybridisation and shape given in column II and mark the correct option. [NEET - 2016]
Column-I Column -II [a] \[Xe{{F}_{6}}\] (i) Distorted octahedral [b] \[Xe{{O}_{3}}\] (ii) Square planar [c] \[XeO{{F}_{4}}\] (iii) Pyramidal [d] \[Xe{{F}_{4}}\] (iv) Square pyramidal
Code :-
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 81) The pair of electron in the given carbanion, \[C{{H}_{3}}C\equiv {{C}^{\,O-}},\] is present in which of the following orbitals? [NEET - 2016]
A)
2p
done
clear
B)
\[s{{p}^{3}}\]
done
clear
C)
\[s{{p}^{2}}\]
done
clear
D)
sp
done
clear
View Answer play_arrow
question_answer 82) Which of the following pairs of compounds is isoelectronic and isostructural? [NEET-2017]
A)
\[I{{F}_{3}},Xe{{F}_{2}}\]
done
clear
B)
\[BeC{{l}_{2}},Xe{{F}_{2}}\]
done
clear
C)
\[Te{{l}_{2}},Xe{{F}_{2}}\]
done
clear
D)
\[IBr_{2}^{-},Xe{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 83)
Match the interhalogen compounds of column I with the geometry in column II and assign the correct code [NEET-2017] Column-I Column -II [a] \[XX'\] (i) T-shape [b] \[XX_{3}^{'}\] (ii) Pentagonal bipyramidal [c] \[XX_{5}^{'}\] (iii) Linear [d] \[XX_{7}^{'}\] (iv) Square-pyramidal (v) Tetrahedral
Code:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 84) The species, having bond angles of \[{{120}^{o}}\] is [NEET-2017]
A)
\[BC{{l}_{3}}\]
done
clear
B)
\[P{{H}_{3}}\]
done
clear
C)
\[CI{{F}_{3}}\]
done
clear
D)
\[NC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 85) Which one of the following pairs of species have the same bond order? [NEET-2017]
A)
\[{{N}_{2}}O_{2}^{-}\]
done
clear
B)
\[CO,NO\]
done
clear
C)
\[{{O}_{2}},N{{O}^{+}}\]
done
clear
D)
\[C{{N}^{-}},CO\]
done
clear
View Answer play_arrow
question_answer 86) In the structure of \[\text{Cl}{{\text{F}}_{\text{3}}}\], the number of lone pair of electrons on central atom \[\text{ }\!\!'\!\!\text{ Cl }\!\!'\!\!\text{ }\] is [NEET - 2018]
A)
Four
done
clear
B)
Two
done
clear
C)
One
done
clear
D)
Three
done
clear
View Answer play_arrow
question_answer 87) Which of the following molecules represents the order of hybridisation \[\text{s}{{\text{p}}^{\text{2}}}\text{, s}{{\text{p}}^{\text{2}}}\text{, sp, sp}\]from left to right atoms? [NEET - 2018]
A)
\[\text{C}{{\text{H}}_{\text{2}}}\text{=CH-CH=C}{{\text{H}}_{\text{2}}}\]
done
clear
B)
\[\text{C}{{\text{H}}_{\text{2}}}\text{=CH-C}\equiv \text{CH}\]
done
clear
C)
\[\text{HC}\equiv \text{C-C}\equiv \text{CH}\]
done
clear
D)
\[\text{C}{{\text{H}}_{\text{3}}}\text{-CH=CH-C}{{\text{H}}_{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 88)
Consider the following species : \[\text{C}{{\text{N}}^{\text{+}}}\text{, C}{{\text{N}}^{\text{--}}}\text{, NO and CN}\] Which one of these will have the highest bond order? [NEET - 2018]
A)
\[\text{C}{{\text{N}}^{\text{+}}}\]
done
clear
B)
\[\text{C}{{\text{N}}^{\text{--}}}\]
done
clear
C)
NO
done
clear
D)
CN
done
clear
View Answer play_arrow
question_answer 89) Magnesium reacts with an element (X) to form an ionic compound. If the ground state electronic configuration of (X) is \[1{{s}^{2}}\text{ }2{{s}^{2}}\text{ }2{{p}^{3}}\], the simplest formula for this compound is [NEET - 2018]
A)
\[\text{M}{{\text{g}}_{\text{2}}}\text{X}\]
done
clear
B)
\[\text{Mg}{{\text{X}}_{2}}\]
done
clear
C)
\[\text{M}{{\text{g}}_{2}}{{\text{X}}_{3}}\]
done
clear
D)
\[\text{M}{{\text{g}}_{3}}{{\text{X}}_{2}}\]
done
clear
View Answer play_arrow
question_answer 90) Which of the following diatomic molecular species has only \[\pi \] bonds according to Molecular Orbital Theory? [NEET 2019]
A)
\[{{C}_{2}}\]
done
clear
B)
\[B{{e}_{2}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 91) Which of the following set of molecules will have zero dipole moment? [NEET 2020]
A)
Boron trifluoride, hydrogen fluoride, carbon dioxide, 1, 3-dichlorobenzene
done
clear
B)
Nitrogen trifluoride, beryllium difluoride, water, 1, 3-dichlorobenzene
done
clear
C)
Boron trifluoride, beryllium difluoride, carbon dioxide, 1, 4-dichlorobenzene
done
clear
D)
Ammonia, beryllium difluoride, water, 1, 4-dichlorobenzene
done
clear
View Answer play_arrow
question_answer 92) Identify a molecule which does not exist. [NEET 2020]
A)
\[L{{i}_{2}}\]
done
clear
B)
\[{{C}_{2}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[H{{e}_{2}}\]
done
clear
View Answer play_arrow