question_answer 1) The twinkling effect of star light is due to
A)
total internal reflection
done
clear
B)
high dense matter of star
done
clear
C)
constant burning of hydrogen in the star
done
clear
D)
the fluctuating apparent position of the star being slightly different from the actual position of the star
done
clear
View Answer play_arrow
question_answer 2) The width of the diffraction band varies
A)
inversely as the wavelength
done
clear
B)
directly as the width of the slit
done
clear
C)
directly as the distance between the slit and the screen
done
clear
D)
inversely as the size of the source from which the slit is illuminated
done
clear
View Answer play_arrow
question_answer 3) An unpolarised beam of intensity \[{{\text{I}}_{\text{o}}}\]is incident on a pair of nicols making an angle of \[{{60}^{o}}\] with each other. The intensity of light emerging from the pair is
A)
\[{{I}_{o}}\]
done
clear
B)
\[\frac{{{I}_{o}}}{2}\]
done
clear
C)
\[\frac{{{I}_{o}}}{4}\]
done
clear
D)
\[\frac{{{I}_{o}}}{8}\]
done
clear
View Answer play_arrow
question_answer 4) When a low flying aircraft passes over head, we sometimes notice a slight shaking of the picture on our TV screen. This is due to
A)
diffraction of the signal received from the antenna
done
clear
B)
interference of the direct signal received by the antenna with the weak signal reflected by the passing aircraft
done
clear
C)
change of magnetic flux occurring due to the passage of aircraft
done
clear
D)
vibration created by the passage of aircraft
done
clear
View Answer play_arrow
question_answer 5) A beam of light of wavelength 600 nm from a distant source falls on a single slit I mm wide and the resulting diffraction-pattern is observed on a screen 2m away. The distance between the first dark fringes on either side of the central bright fringe is
A)
1.2 cm
done
clear
B)
1.2 mm
done
clear
C)
2.4cm
done
clear
D)
2.4mm
done
clear
View Answer play_arrow
question_answer 6) The physical quantity having the dimensions \[[{{M}^{-1}}{{L}^{-3}}{{T}^{3}}{{A}^{2}}]\] is
A)
resistance
done
clear
B)
resistivity
done
clear
C)
electrical conductivity
done
clear
D)
electromotive force
done
clear
View Answer play_arrow
question_answer 7) A battery of emf 10 V and internal resistance \[3\,\Omega \]is connected to a resistor. The current in the circuit is 0.5 A. The terminal voltage of the battery when the circuit is closed is
A)
\[10\,V\]
done
clear
B)
\[0\,V\]
done
clear
C)
\[1.5\,V\]
done
clear
D)
\[8.5\,V\]
done
clear
View Answer play_arrow
question_answer 8) A galvanometer coil has a resistance of \[15\,\Omega \] and gives full scale deflection for a current of 4 mA. To convert it to an ammeter of range 0 to 6 A
A)
\[10\,m\Omega \]resistance is to be connected in parallel to the galvanometer
done
clear
B)
\[10\,m\Omega \] resistance is to be connected in series with the galvanometer
done
clear
C)
\[0.1\,\Omega \]resistance is to be connected in parallel to the galvanometer
done
clear
D)
\[0.1\,\Omega \]resistance is to be connected in series with the galvanometer
done
clear
View Answer play_arrow
question_answer 9) The electron drift speed is small and the charge of the electron is also small but still, we obtain large current in a conductor. This is due to
A)
the conducting property of the conductor
done
clear
B)
the resistance of the conductor is small
done
clear
C)
the electron number density of the conductor is small
done
clear
D)
the electron number density of the conductor is enormous
done
clear
View Answer play_arrow
question_answer 10) A straight wire of mass 200 g and length 1.5 m carries a current of 2 A. It is suspended in mid-air by a uniform horizontal magnetic field B. The magnitude of B (in testa) is (assume\[g=9.8\,m{{s}^{-2}}\])
A)
2
done
clear
B)
1.5
done
clear
C)
0.55
done
clear
D)
0.65
done
clear
View Answer play_arrow
question_answer 11) A Gaussian sphere encloses an electric dipole within it. The total flux across the sphere is
A)
zero
done
clear
B)
half that due to a single charge
done
clear
C)
double that due to a single charge
done
clear
D)
dependent on the position of the dipole
done
clear
View Answer play_arrow
question_answer 12) A parallel plate air capacitor has a capacitance C. When it is half filled with a dielectric of dielectric constant 5, the percentage increase in the capacitance will be
A)
400%
done
clear
B)
66.6%
done
clear
C)
33.3%
done
clear
D)
200%
done
clear
View Answer play_arrow
question_answer 13) A comb run through ones dry hair attracts small bits of paper. This is due to
A)
comb is a good conductor
done
clear
B)
paper is a good conductor
done
clear
C)
the atoms is the paper get polarised by the charged comb
done
clear
D)
the comb possesses magnetic properties
done
clear
View Answer play_arrow
question_answer 14) The specific charge of a proton is \[9.6\times {{10}^{7}}C\,k{{g}^{-1}}.\] The specific charge of an alpha particle will be
A)
\[9.6\times {{10}^{7}}C\,k{{g}^{-1}}\]
done
clear
B)
\[19.2\times {{10}^{7}}C\,k{{g}^{-1}}\]
done
clear
C)
\[4.8\times {{10}^{7}}C\,k{{g}^{-1}}\]
done
clear
D)
\[2.4\times {{10}^{7}}C\,k{{g}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 15) When light of wavelength 300 nm falls on a photoelectric emitter, photoelectrons are liberated. For another, emitter, light of wavelength 600 nm is sufficient for liberating photoelectrons. The ratio of the work function of the two emitters is
A)
1:2
done
clear
B)
2:1
done
clear
C)
4:1
done
clear
D)
1:4
done
clear
View Answer play_arrow
question_answer 16) White light is passed through a dilute solution of potassium permanganate. The spectrum produced by the emergent light is
A)
band emission spectrum
done
clear
B)
line emission spectrum
done
clear
C)
band absorption spectrum
done
clear
D)
line absorption spectrum
done
clear
View Answer play_arrow
question_answer 17) If\[{{\lambda }_{1}}\] and\[{{\lambda }_{2}}\] are the wavelengths of the first members of the Lyman and Paschen series respectively, then \[{{\lambda }_{1}}:{{\lambda }_{2}}\]is
A)
1 : 3
done
clear
B)
1 : 30
done
clear
C)
7 : 50
done
clear
D)
7 : 108
done
clear
View Answer play_arrow
question_answer 18) Activity of a radioactive sample decreases to \[{{(1/3d)}^{rd}}\]of its original value in 3 days. Then, in 9 days its activity will become
A)
(1/27) of the original value
done
clear
B)
(1/9) of the original value
done
clear
C)
(1/18) of the original value
done
clear
D)
(1/3) of the original value
done
clear
View Answer play_arrow
question_answer 19) The working of which of the following is similar to that of a slide projector?
A)
Electron microscope
done
clear
B)
Scanning electron microscope
done
clear
C)
Transmission electron microscope
done
clear
D)
Atomic force microscope
done
clear
View Answer play_arrow
question_answer 20) In a transistor the collector current is always less than the emitter current bacause
A)
collector side is reverse biased and the emitter side is forward biased
done
clear
B)
a few electrons are lost in the base and only remaining ones reach the collector
done
clear
C)
collector being reverse/biased, attracts less electrons
done
clear
D)
collector side is forward biased and emitter side is reverse biased
done
clear
View Answer play_arrow
question_answer 21) A transparent cube of 0.21 m edge contains a small air bubble. Its apparent distance when viewed through one face of the cube is 0.10 m and when viewed from the opposite face is 0.04 m. The actual distance of the bubble from the second face of the cube is
A)
0.06 m
done
clear
B)
0.17 m
done
clear
C)
0.05 m
done
clear
D)
0.04 m
done
clear
View Answer play_arrow
question_answer 22) To a fish under water, viewing obliquely a fisherman standing on the bank of a lake, the man looks
A)
taller than what he actually is
done
clear
B)
shorter that what he actually is
done
clear
C)
the same height as he actually is
done
clear
D)
depends on the obliquity
done
clear
View Answer play_arrow
question_answer 23) If white light is used in the Newtons rings experiment, the colour observed in the reflected light is complementary to that observed in the transmitted light through the same point. This is due to
A)
\[{{90}^{o}}\]change of phase in one of the reflected waves
done
clear
B)
\[{{180}^{o}}\]change of phase in one of the reflected waves
done
clear
C)
\[{{145}^{o}}\]change of phase in one of the reflected waves
done
clear
D)
\[{{45}^{o}}\]change of phase in one the reflected waves
done
clear
View Answer play_arrow
question_answer 24) A satellite in a circular orbit of radius R has a period of 4 h. Another satellite with orbital radius 3R around the same planet will have a period (in hours)
A)
16
done
clear
B)
4
done
clear
C)
\[4\sqrt{27}\]
done
clear
D)
\[4\sqrt{8}\]
done
clear
View Answer play_arrow
question_answer 25) The freezer in a refrigerator is located at the top section so that
A)
the entire chamber of the refrigerator is cooled quickly due to convection
done
clear
B)
the motor is not heated
done
clear
C)
the heat gained from the environment is high
done
clear
D)
the heat gained from the environment is low
done
clear
View Answer play_arrow
question_answer 26) A monoatomic gas is suddenly compressed to \[{{\text{(1/8)}}^{\text{th}}}\]of its initial volume adiabatically. The ratio of its final pressure to the initial pressure is (Given the ratio of the specific heats of the given gas to be 5/3)
A)
32
done
clear
B)
40/3
done
clear
C)
24/5
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 27) A Camot engine takes heat from a reservoir at \[\text{627}{{\,}^{\text{o}}}\text{C}\]and rejects heat to a sink at \[\text{27}{{\,}^{o}}\text{C}\text{.}\] Its efficiency will be
A)
3/5
done
clear
B)
1/3
done
clear
C)
2/3
done
clear
D)
200/209
done
clear
View Answer play_arrow
question_answer 28) A 30 V, 90 W lamp is to be operated on a 120 V DC line. For proper glow, a resistor of ... \[\Omega \] should be connected in series with the lamp.
A)
40
done
clear
B)
10
done
clear
C)
20
done
clear
D)
30
done
clear
View Answer play_arrow
question_answer 29) A tuning fork A produces 4 beats/s with another tuning fork B of frequency 320 Hz. On filing one of the prongs of A, 4 beats/s are again heard when sounded with the same fork B. Then, the frequency of the fork A before filing is
A)
328 Hz
done
clear
B)
316 Hz
done
clear
C)
324 Hz
done
clear
D)
320 Hz
done
clear
View Answer play_arrow
question_answer 30) The sprinkling of water reduces slightly the temperature of a closed room because
A)
temperature of water is less than that of the room
done
clear
B)
specific heat of water is high
done
clear
C)
water has large latent heat of vaporization
done
clear
D)
water is a bad conductor of heat
done
clear
View Answer play_arrow
question_answer 31) The equation of a simple harmonic wave is given by \[y=5\sin \frac{\pi }{2}(100t-x),\]where \[x\]and \[y\]are in metre and time is in second. The period of the wave in second will be
A)
0.04
done
clear
B)
0.01
done
clear
C)
1
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 32) The loudness and pitch of a sound note depends on
A)
intensity and frequency
done
clear
B)
frequency and number of harmonics
done
clear
C)
intensity and velocity
done
clear
D)
frequency and velocity
done
clear
View Answer play_arrow
question_answer 33) For ordinary terrestrial experiments, the observer in an inertial frame in the following cases is
A)
a child revolving in a giant wheel
done
clear
B)
a driver in a sports car moving with a constant high speed of \[200\,\text{km}{{\text{h}}^{-1}}\] on a straight rod
done
clear
C)
the pilot of an aeroplane which is taking off
done
clear
D)
a cyclist negotiating a sharp curve
done
clear
View Answer play_arrow
question_answer 34) A rectangular vessel when full of water, takes 10 min to be emptied through an orifice in its bottom. How much time will it take to be emptied when half filled with water?
A)
9 min
done
clear
B)
7 min
done
clear
C)
5 min
done
clear
D)
3 min
done
clear
View Answer play_arrow
question_answer 35) If there were no gravity, which of the following will not be there for a fluid?
A)
Viscosity
done
clear
B)
Surface tension
done
clear
C)
Pressure
done
clear
D)
Archimedes upward thrust
done
clear
View Answer play_arrow
question_answer 36) In a LCR series circuit, the potential difference between the terminals of the inductance is 60 V, between the terminals of the capacitor is 30 V and that across the resistance is 40 V. Then, supply voltage will be equal to
A)
50 V
done
clear
B)
70 V
done
clear
C)
130 V
done
clear
D)
10 V
done
clear
View Answer play_arrow
question_answer 37) When deuterium and helium are subjected to an accelerating field simultaneously then
A)
both acquire same energy
done
clear
B)
deuterium accelerates faster
done
clear
C)
helium accelerates faster
done
clear
D)
neither of them is accelerated
done
clear
View Answer play_arrow
question_answer 38) A solenoid 1.5 m long and 0.4 cm in diameter possesses 10 turns per cm length. A current of 5 A falls through it. The magnetic field at the axis inside the solenoid is
A)
\[2\pi \times {{10}^{-3}}T\]
done
clear
B)
\[2\pi \times {{10}^{-5}}T\]
done
clear
C)
\[4\pi \times {{10}^{-2}}T\]
done
clear
D)
\[4\pi \times {{10}^{-3}}T\]
done
clear
View Answer play_arrow
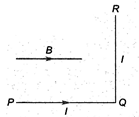
question_answer 39)
A wire PQR is bent as shown in figure and is placed in a region of uniform magnetic field B. The length of\[PQ=QR=l.A\] current \[I\] ampere flows through the wire as shown. The agnitude of the force on PQ and QR will be
A)
\[BIl,0\]
done
clear
B)
\[2BIl,0\]
done
clear
C)
\[0,BIl\]
done
clear
D)
\[0,0\]
done
clear
View Answer play_arrow
question_answer 40) A choke is preferred to a resistance for limiting current in AC circuit because
A)
choke is cheap
done
clear
B)
there is no wastage of power
done
clear
C)
choke is compact in size
done
clear
D)
choke is a good absorber of heat
done
clear
View Answer play_arrow
question_answer 41) If \[{{r}_{1}}\]and \[{{r}_{2}}\]are the radii of the atomic nuclei of mass numbers 64 and 125 respectively, then the ratio \[({{r}_{1}}/{{r}_{2}})\]is
A)
\[\frac{64}{125}\]
done
clear
B)
\[\sqrt{\frac{64}{125}}\]
done
clear
C)
\[\frac{5}{5}\]
done
clear
D)
\[\frac{4}{5}\]
done
clear
View Answer play_arrow
question_answer 42) A motor is used to deliver water at a certain rate through a given horizontal pipe. To deliver \[n-\]fimes the water through the same pipe in the same time the power of the motor must be increased as follows
A)
\[n-\]times
done
clear
B)
\[{{n}^{2}}-\] times
done
clear
C)
\[{{n}^{3}}-\]times
done
clear
D)
\[{{n}^{4}}-\]times
done
clear
View Answer play_arrow
question_answer 43) Fora system to follow the law of conservation of linear momentum during a collision, the condition is (i) total external force acting on the system is zero (ii) total external force acting oh the system is finite and time of collision is negligible. (iii) total internal force acting on the system is zero.
A)
(i)only
done
clear
B)
(ii) only
done
clear
C)
(iii) only
done
clear
D)
(i)or(ii)
done
clear
View Answer play_arrow
question_answer 44) An air bubble of radius 1 cm rises from the bottom portion through a liquid of density \[1.5~g/cc\]at a constant speed of \[0.25\,cm\,{{s}^{-1}}.\]If the density of air is neglected, the coefficient of viscosity of the liquid is approximately (in Pa)
A)
13000
done
clear
B)
1300
done
clear
C)
130
done
clear
D)
13
done
clear
View Answer play_arrow
question_answer 45) A given mass of a gas is compressed isothermally until its pressure is doubled. It is then allowed to expand adiabatically until its original volume is restored and its pressure is then found to be 0.75 of its initial pressure. The ratio of the specific heats of the gas is approximately
A)
1.20
done
clear
B)
1.41
done
clear
C)
1.67
done
clear
D)
1.83
done
clear
View Answer play_arrow
question_answer 46) Two solid spheres A and B made of the same material have radii\[{{r}_{A}}\] and \[{{r}_{B}}\] respectively. Both the spheres are cooled from the same temperature under the conditions valid for Newtons law of cooling. The ratio of the rate of change of temperature A and B is
A)
\[\frac{{{r}_{A}}}{{{r}_{B}}}\]
done
clear
B)
\[\frac{{{r}_{B}}}{{{r}_{A}}}\]
done
clear
C)
\[\frac{r_{A}^{2}}{r_{B}^{2}}\]
done
clear
D)
\[\frac{r_{B}^{2}}{r_{A}^{2}}\]
done
clear
View Answer play_arrow
question_answer 47) The effect due to uniform magnetic field on a freely suspended magnetic needle is as follows
A)
both torque and net force are present
done
clear
B)
torque is present but no net force
done
clear
C)
both torque and net force are absent
done
clear
D)
net force is present but not torque
done
clear
View Answer play_arrow
question_answer 48) When a positively charged particle enters a uniform magnetic field with uniform velocity, its trajectory can be (i) a straight line (ii) a circle (iii) a helix
A)
(i) only
done
clear
B)
(i)pr(ii)
done
clear
C)
(i) or (iii)
done
clear
D)
anyone of (i), (ii) and (iii)
done
clear
View Answer play_arrow
question_answer 49) A oil drop having a mass \[4.8\times {{10}^{-10}}\]and charge \[24\times {{10}^{-18}}C\] stands still between two charged horizontal plates separated by a distance of 1 cm. If now the polarity of the plates is changed, instantaneous acceleration of the drop is ( \[g=10\,m{{s}^{-2}}\])
A)
\[5m{{s}^{-2}}\]
done
clear
B)
\[10\,m{{s}^{-2}}\]
done
clear
C)
\[15\,m{{s}^{-2}}\]
done
clear
D)
\[20\,m{{s}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 50) A free neutron decays spontaneously into
A)
a proton, an electron and anti-neutrino
done
clear
B)
a proton, an electron and a neutrino
done
clear
C)
a proton and electron
done
clear
D)
a proton, and electron, a neutrino and an anti-neutrino
done
clear
View Answer play_arrow
question_answer 51) What is the correct order of spin only magnetic moment (in BM) of \[M{{n}^{2+}},C{{r}^{2+}}\]and \[{{V}^{2+}}\]?
A)
\[M{{n}^{2+}}>{{V}^{2+}}>C{{r}^{2+}}\]
done
clear
B)
\[{{V}^{2+}}>C{{r}^{2+}}>M{{n}^{2+}}\]
done
clear
C)
\[M{{n}^{2+}}>C{{r}^{2+}}>{{V}^{2+}}\]
done
clear
D)
\[C{{r}^{2+}}>{{V}^{2+}}>M{{n}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 52) Which of the following is used for making optical instruments?
A)
\[Si{{O}_{2}}\]
done
clear
B)
\[Si\]
done
clear
C)
\[Si{{H}_{4}}\]
done
clear
D)
\[SiC\]
done
clear
View Answer play_arrow
question_answer 53) Which of the following is not correct?
A)
\[3{{O}_{2}}2{{O}_{3}};\Delta H=-284.5\,kJ\]
done
clear
B)
Ozone undergoes addition reaction with unsaturated carbon compounds
done
clear
C)
Sodium thiosulphate reacts with \[{{I}_{2}}\]to form sodium tetrathionate and sodium iodide
done
clear
D)
Ozone oxidises lead sulphide to lead sulphate
done
clear
View Answer play_arrow
question_answer 54) Which of the following reactions can produce aniline as main product?
A)
\[{{C}_{6}}{{H}_{5}}NO{{}_{2}}+Zn/KOH\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}N{{O}_{2}}+Zn/N{{H}_{4}}Cl\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}N{{O}_{2}}+LiAl{{H}_{4}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}N{{O}_{2}}+Zn+HCl\]
done
clear
View Answer play_arrow
question_answer 55) Which of the following reagents when heated with ethyl chloride, forms ethylene?
A)
Aqueous KOH
done
clear
B)
\[Zn/HCl\]
done
clear
C)
Alcoholic KOH
done
clear
D)
HI
done
clear
View Answer play_arrow
question_answer 56) The energy of a photon is \[3\times {{10}^{-12}}\text{erg}\text{.}\] What is its wavelength in nm? \[(h=6.62\times {{10}^{-27}}erg\,-s;c=3\times {{10}^{10}}cm/s)\]
A)
662
done
clear
B)
1324
done
clear
C)
66.2
done
clear
D)
6.62
done
clear
View Answer play_arrow
question_answer 57) What is the time (in sec) required for depositing all the silver present in 125 mL of \[\text{1}\,\text{M}\,\text{AgN}{{\text{O}}_{\text{3}}}\]solution by passing a current of 241.25 A?\[\text{(1F}\,\text{=}\,\text{96500}\,\text{C)}\]
A)
10
done
clear
B)
50
done
clear
C)
1000
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 58) The disperse phase, dispersion medium and nature of colloidal solution (lyophilic or lyophobic) of gold sol respectively, are
A)
Solid, solid, lyophobic
done
clear
B)
liquid, liquid, lyophobic
done
clear
C)
solid, liquid, lyophobic
done
clear
D)
solid, liquid, lyophilic
done
clear
View Answer play_arrow
question_answer 59) The rate constant of a first order reaction at \[\text{27}{{\,}^{o}}\text{C}\]is \[{{10}^{-3}}{{\min }^{-1}}.\]The temperature coefficient of this reaction is 2. What is the rate constant (in\[{{\min }^{-1}}\]) at \[17{{\,}^{o}}C\]for this reaction?
A)
\[{{10}^{-3}}\]
done
clear
B)
\[5\times {{10}^{-4}}\]
done
clear
C)
\[2\times {{10}^{-3}}\]
done
clear
D)
\[{{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 60) A solution of an acid has \[[{{H}^{+}}]=2\times {{10}^{-5}}.\]Find out the concentration of \[\text{O}{{\text{H}}^{-}}\]ions.
A)
\[5\times {{10}^{-10}}N\]
done
clear
B)
\[4\times {{10}^{-10}}N\]
done
clear
C)
\[2\times {{10}^{-5}}N\]
done
clear
D)
\[9\times {{10}^{-4}}N\]
done
clear
View Answer play_arrow
question_answer 61) Which of the followingjs added to chloroform to slow down its aerial oxidation in presence of light
A)
Carbonyl chloride
done
clear
B)
Ethylalcohol
done
clear
C)
Sodium hydroxide
done
clear
D)
Nitric acid
done
clear
View Answer play_arrow
question_answer 62) Which of the products is formed when acetone is reacted with barium hydroxide solution?
A)
\[C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{2}}-\underset{OH}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-\underset{OH}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-\underset{OH}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-\underset{C{{H}_{3}}}{\overset{OH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{C{{H}_{3}}}{\overset{OH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 63) When acetaldehyde is heated with Fehling solution, a red precipitate is formed. Which of the following is that?
A)
\[C{{u}_{2}}O\]
done
clear
B)
\[Cu\]
done
clear
C)
\[CuO\]
done
clear
D)
\[CuS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 64) What is the correct order of occurrence (% by weight) in air of Ne, Ar and Kr?
A)
\[Ne>Ar>Kr\]
done
clear
B)
\[Ar>Ne>Kr\]
done
clear
C)
\[Ar>Kr>Ne\]
done
clear
D)
\[Ne>Kr>Ar\]
done
clear
View Answer play_arrow
question_answer 65) Which of the following compounds when heated with CO at \[\text{150}{{\,}^{\text{o}}}\text{C}\]and 500 atm pressure in presence\[\text{B}{{\text{F}}_{\text{3}}}\]forms ethyl propionate?
A)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[C{{H}_{3}}OC{{H}_{3}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\]
done
clear
D)
\[C{{H}_{3}}O{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 66) Identify the reaction for which \[\Delta H\ne \Delta E\].
A)
\[S(rhombic)+{{O}_{2}}(g)\xrightarrow{{}}S{{O}_{2}}(g)\]
done
clear
B)
\[{{N}_{2}}(g)+{{O}_{2}}(g)\xrightarrow{{}}2NO(g)\]
done
clear
C)
\[{{H}_{2}}(g)+C{{l}_{2}}(g)\xrightarrow{{}}2HCl(g)\]
done
clear
D)
\[CO(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}C{{O}_{2}}(g)\]
done
clear
View Answer play_arrow
question_answer 67) Hydrolysis of \[\text{NC}{{\text{l}}_{\text{3}}}\]gives \[\text{N}{{\text{H}}_{3}}\]and X. Which of the following is X?
A)
\[HCl{{O}_{4}}\]
done
clear
B)
\[HCl{{O}_{3}}\]
done
clear
C)
\[HOCl\]
done
clear
D)
\[HCl{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 68) What are the metal ions present in camallite?
A)
\[Mg,K\]
done
clear
B)
\[Al,Na\]
done
clear
C)
\[Na,Mg\]
done
clear
D)
\[Zn,Mg\]
done
clear
View Answer play_arrow
question_answer 69) Ethyl chloride reacts with sodium ethoxide to form a compound A. Which of the following reactions also yields A?
A)
\[{{C}_{2}}{{H}_{5}}Cl,KOH(alc),\Delta \]
done
clear
B)
\[2{{C}_{2}}{{H}_{5}}OH,conc.{{H}_{2}}S{{O}_{4}},140{{\,}^{o}}C\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}Cl,Mg\](dry ether)
done
clear
D)
\[{{C}_{2}}{{H}_{2}}\text{dil}\text{.}\,{{H}_{2}}S{{O}_{4}},HgS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 70) The number of sigma and pi\[(\pi )\] bonds present in benzene respectively are
A)
12, 6
done
clear
B)
6, 6
done
clear
C)
6, 12
done
clear
D)
12, 3
done
clear
View Answer play_arrow
question_answer 71) Edge length of a cube is 400 pm, its body diagonal would be
A)
566 pm
done
clear
B)
600 pm
done
clear
C)
500 pm
done
clear
D)
693 pm
done
clear
View Answer play_arrow
question_answer 72) The number of \[\alpha -\]particles emitted by \[{{\,}_{84}}R{{a}^{218}}\xrightarrow{{}}{{\,}_{82}}P{{b}^{206}}\]is
A)
3
done
clear
B)
4
done
clear
C)
6
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 73) The IUPAC name of the following compound is \[C{{H}_{3}}-\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{2}}-C{{H}_{3}}\]
A)
2-cyclohexylbutane
done
clear
B)
sec-butylbenzene
done
clear
C)
3-cyclohexylbutane
done
clear
D)
2-phenylbutane
done
clear
View Answer play_arrow
question_answer 74) The reaction of primary amine with chloroform and ethanolic solution of KOH is called
A)
Hofmanns reaction
done
clear
B)
Reimer-Tiemanns reaction
done
clear
C)
Carbylamine reaction
done
clear
D)
Kolbes reaction
done
clear
View Answer play_arrow
question_answer 75) 0.01 mole of a non-electrolyte is dissolved in 10 g of water. The molality of the solution is
A)
0.1 m
done
clear
B)
0.5 m
done
clear
C)
1.0 m
done
clear
D)
0.18 m
done
clear
View Answer play_arrow
question_answer 76) Atoms with same atomic number and different mass numbers are called
A)
isobars
done
clear
B)
isomers
done
clear
C)
isotones
done
clear
D)
isotopes
done
clear
View Answer play_arrow
question_answer 77) The shape of the orbital with the value of \[l=2\]and \[m=0\]is
A)
spherical
done
clear
B)
dumb-bell
done
clear
C)
trigonal planar
done
clear
D)
square-planar
done
clear
View Answer play_arrow
question_answer 78) In the following, the element with the highest ionisation energy is
A)
\[[Ne]3{{s}^{2}}3{{p}^{1}}\]
done
clear
B)
\[[Ne]3{{s}^{2}}3{{p}^{3}}\]
done
clear
C)
\[[Ne]3{{s}^{2}}3{{p}^{2}}\]
done
clear
D)
\[[Ne]3{{s}^{2}}3{{p}^{4}}\]
done
clear
View Answer play_arrow
question_answer 79) In the conversion of \[B{{r}_{2}}\]to \[BrO_{3}^{-},\]the oxidation number of Br changes from
A)
\[\text{zero to}+5\]
done
clear
B)
\[+\,1\,\text{to}\,\text{+}\,\text{5}\]
done
clear
C)
\[\text{ }\!\!~\!\!\text{ zero to}-3\]
done
clear
D)
\[~+\,2\text{ to}+5\]
done
clear
View Answer play_arrow
question_answer 80) Among the alkali metals cesium is the most reactive because
A)
its incomplete shell is nearest to the nucleus
done
clear
B)
it has a single electron in the valence shell
done
clear
C)
it is the heaviest alkali metal
done
clear
D)
the outermost electron is more loosely bound than the outermost electron of the other alkali metals
done
clear
View Answer play_arrow
question_answer 81) Which of the following represents the Lewis structure of \[{{\text{N}}_{\text{2}}}\]molecule?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 82) Hydrogen bond is strongest in
A)
\[S-H...O\]
done
clear
B)
\[O-H...S\]
done
clear
C)
\[F-H...F\]
done
clear
D)
\[O-H...N\]
done
clear
View Answer play_arrow
question_answer 83) The density of a gas is \[1.964\,g\,d{{m}^{-3}}\] at 273 K and 76cmHg. The gas is
A)
\[C{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[Xe\]
done
clear
View Answer play_arrow
question_answer 84) The shape of \[\text{PC}{{\text{l}}_{\text{3}}}\]molecule is
A)
trigonal bipyramidal
done
clear
B)
tetrahedral
done
clear
C)
pyramidal
done
clear
D)
square planar
done
clear
View Answer play_arrow
question_answer 85) The concentration of a reactant X decreases from 0.1 M to 0.005 M in 40 min. If the reaction follows first order kinetics, the rate of the reaction when the concentration of X is 0.01 M will be
A)
\[1.73\,\times {{10}^{-4}}\,M{{\min }^{-1}}\]
done
clear
B)
\[3.47\times {{10}^{-4}}M\,{{\min }^{-1}}\]
done
clear
C)
\[3.47\times {{10}^{-5}}M\,{{\min }^{-1}}\]
done
clear
D)
\[7.5\times {{10}^{-4}}M\,{{\min }^{-1}}\]
done
clear
View Answer play_arrow
question_answer 86) Which of the following does not conduct electricity?
A)
Fused NaCI
done
clear
B)
Solid NaCI
done
clear
C)
Brine solution
done
clear
D)
Copper
done
clear
View Answer play_arrow
question_answer 87) Solubility product of a salt AB is \[1\times {{10}^{-8}}{{M}^{2}}\,\]in a solution in which the concentration of \[{{A}^{+}}\]ions is \[{{10}^{-3}}\,M.\]The salt will precipitate when the concentration of \[{{B}^{-}}\]ions is kept
A)
between \[{{10}^{-8}}\,M\]to \[{{10}^{-7}}\,M\]
done
clear
B)
between \[{{10}^{-7}}M\]to \[{{10}^{-8}}M\]
done
clear
C)
\[>{{10}^{-5}}M\]
done
clear
D)
\[<{{10}^{-8}}M\]
done
clear
View Answer play_arrow
question_answer 88) The pH of \[{{10}^{-8}}\,M\,HCl\]solution is
A)
8
done
clear
B)
more than 8
done
clear
C)
between 6 and 7
done
clear
D)
slightly more than 7
done
clear
View Answer play_arrow
question_answer 89) For a reaction to be spontaneous at all temperatures
A)
\[\Delta G\]and \[\Delta H\]should be negative
done
clear
B)
\[\Delta G\]and\[\Delta H\] should be positive
done
clear
C)
\[\Delta G=\Delta S=0\]
done
clear
D)
\[\Delta H=\Delta G\]
done
clear
View Answer play_arrow
question_answer 90) Which of the following electrolytes will have maximum flocculation value for \[Fe{{(OH)}_{3}}sol\]
A)
\[NaCl\]
done
clear
B)
\[N{{a}_{2}}S\]
done
clear
C)
\[{{(N{{H}_{4}})}_{3}}P{{O}_{4}}\]
done
clear
D)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 91) What is the order of a reaction which has, a rate expression \[rate=k{{[A]}^{3/2}}{{[B]}^{-1}}?\]
A)
\[\frac{3}{2}\]
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
0
done
clear
D)
\[\frac{4}{2}\]
done
clear
View Answer play_arrow
question_answer 92) Inductive effect involves
A)
displacement of o-electrons
done
clear
B)
delocalisation of Ti-electrons
done
clear
C)
delocalisation of o-electrons
done
clear
D)
displacement of Ti-electrpns
done
clear
View Answer play_arrow
question_answer 93) Which of the following compound is expected to be optically active?
A)
\[{{(C{{H}_{3}})}_{2}}CHCHO\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CHO\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CHBrCHO\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}CB{{r}_{2}}CHO\]
done
clear
View Answer play_arrow
question_answer 94) The catalyst used in the preparation of an alkyl chloride by the action of dry HC1 on an alcohol is
A)
anhydrous \[AlC{{l}_{3}}\]
done
clear
B)
\[FeC{{l}_{3}}\]
done
clear
C)
anhydrous\[ZnC{{l}_{2}}\]
done
clear
D)
Cu
done
clear
View Answer play_arrow
question_answer 95) By heating phenol with chloroform in alkali, it is converted into
A)
salicylic acid
done
clear
B)
salicylaldehyde
done
clear
C)
anisole
done
clear
D)
phenyl benzoate
done
clear
View Answer play_arrow
question_answer 96) Which of the following does not give benzoic acid on hydrolysis?
A)
Phenyl cyanide
done
clear
B)
Benzoyl chloride
done
clear
C)
Benzyl chloride
done
clear
D)
Methyl benzoate
done
clear
View Answer play_arrow
question_answer 97) Glucose contains in addition to aldehyde group
A)
one secondary-OH and four primary-OH groups
done
clear
B)
one primary-OH and four secondary-OH groups
done
clear
C)
two primary-OH and three secondary-OH groups
done
clear
D)
three primary-OH and two secondary-OH group
done
clear
View Answer play_arrow
question_answer 98) The formula mass of Mohrs salt is 392. The iron present in it is oxidised by \[\text{Kmn}{{\text{O}}_{\text{4}}}\]in acid medium. The equivalent mass of Mohrs salt is
A)
392
done
clear
B)
31.6
done
clear
C)
278
done
clear
D)
156
done
clear
View Answer play_arrow
question_answer 99) The brown ring test for nitrates depends on
A)
the reduction of nitrate to nitric oxide
done
clear
B)
oxidation of nitric oxide to nitrogen dioxide
done
clear
C)
reduction of ferrous sulphate to iron
done
clear
D)
oxidising action of sulphuric acid
done
clear
View Answer play_arrow
question_answer 100) Which of the following solutions will exhibit highest boiling point?
A)
\[\text{0}\text{.01M}\,\text{N}{{\text{a}}_{\text{2}}}S{{O}_{4}}(aq)\]
done
clear
B)
\[\text{0}\text{.01M}\,\text{KN}{{\text{O}}_{\text{3}}}(aq)\]
done
clear
C)
\[0.015\,\text{M}\,\text{urea}\,\text{(aq)}\]
done
clear
D)
\[0.015\,\text{M}\,\text{glucose(aq)}\]
done
clear
View Answer play_arrow
question_answer 101) When \[\text{C}{{\text{O}}_{\text{2}}}\]concentration in blood increases, breathing becomes
A)
shallower and slow
done
clear
B)
there is, no effect on breathing
done
clear
C)
slow and deep
done
clear
D)
faster and deeper
done
clear
View Answer play_arrow
question_answer 102) Cancer cells are more easily damaged by radiation than normal cells because they are
A)
starved of mutation
done
clear
B)
undergoing rapid division
done
clear
C)
different in structure
done
clear
D)
non-dividing
done
clear
View Answer play_arrow
question_answer 103) Which one of the following is not correctly matched?
A)
Glossina palpalis - Sleeping sickness
done
clear
B)
Culex - Filariasis
done
clear
C)
Aedes aegypti - Yellow fever
done
clear
D)
Anopheles culifacies - Leishmaniasis
done
clear
View Answer play_arrow
question_answer 104) A free living nitrogen fixing cyanobacterium which can also form symbiotic association with the water fem Azolla is
A)
Tolypothrix
done
clear
B)
Chlorella
done
clear
C)
Nostoc
done
clear
D)
Anabaena
done
clear
View Answer play_arrow
question_answer 105) Which of the following hormones is not a secretion product of human placenta?
A)
Human chorionic gonadotropin
done
clear
B)
Prolactin
done
clear
C)
Estrogen
done
clear
D)
Progesterone
done
clear
View Answer play_arrow
question_answer 106) The cardiac pace maker in a patient fails to function normally. The doctors find that an artificial pace maker is to be grafted in him. It is likely that it will be grafted at the site of
A)
atrioventricular bundle
done
clear
B)
Plirkinje system
done
clear
C)
sinuatrial node
done
clear
D)
atrioventricular node
done
clear
View Answer play_arrow
question_answer 107) Flagella of prokaryotic and eukaryotic cells differ in
A)
type of movement and placement in cell
done
clear
B)
location in cell and mode of functioning
done
clear
C)
micro-tubular organization and type of movement
done
clear
D)
micro-tubular organization and function
done
clear
View Answer play_arrow
question_answer 108) The animal with bilateral symmetry in young stage, and radial pentamerous symmetry in the adult stage belongs to the phylum
A)
Annelida
done
clear
B)
Mollusca
done
clear
C)
Cnidaria
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow
question_answer 109) Lack of independent assortment of two genes- A and B in fruit fly-Drosophila is due to
A)
repulsion
done
clear
B)
recombination
done
clear
C)
linkage
done
clear
D)
crossing over
done
clear
View Answer play_arrow
question_answer 110) Which of the following is expected to have the highest value \[(gm/{{m}^{2}}/yr)\] in a grassland ecosystem?
A)
Secondary Production (SP)
done
clear
B)
Tertiary Production (TP)
done
clear
C)
Gross Production (GP)
done
clear
D)
Net Production (NP)
done
clear
View Answer play_arrow
question_answer 111) In 1984, the Bhopal gas tragedy took place because methyl isocyanate
A)
reacted with DDT
done
clear
B)
reacted with ammonia
done
clear
C)
reacted with \[\text{C}{{\text{O}}_{\text{2}}}\]
done
clear
D)
reacted with water
done
clear
View Answer play_arrow
question_answer 112) The technique of obtaining large number of plantlets by tissue culture method is called
A)
plantlet culture
done
clear
B)
organ culture
done
clear
C)
micro-propagation
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 113) One set of a plant was grown at 12 hours day and 12 hours night period cycles and it flowered while in the other set night phase was interrupted by flash of light and it did not produce flower. Under which one of the following categories will you place this plant?
A)
Long day
done
clear
B)
Darkness neutral
done
clear
C)
Day neutral
done
clear
D)
Short day
done
clear
View Answer play_arrow
question_answer 114) Which one of the following hormone is a modified amino acid?
A)
Epinephrine
done
clear
B)
Progesterone
done
clear
C)
Prostaglandin
done
clear
D)
Estrogen
done
clear
View Answer play_arrow
question_answer 115) In\[{{\text{C}}_{\text{3}}}\]plants, the first stable product of photosynthesis during the dark reaction is
A)
malic acid
done
clear
B)
oxaloacetic acid
done
clear
C)
3-phosphoglyceric acid
done
clear
D)
phosphoglyceraldehyde
done
clear
View Answer play_arrow
question_answer 116) The maximum growth rate occurs in
A)
stationary phase
done
clear
B)
senescent phase
done
clear
C)
lag phase
done
clear
D)
exponential phase
done
clear
View Answer play_arrow
question_answer 117) Anthesis is a phenomenon which refers to
A)
reception of pollen by stigma
done
clear
B)
formation of pollen
done
clear
C)
development of anther
done
clear
D)
opening of flower bud
done
clear
View Answer play_arrow
question_answer 118) Cell elongation in intemodal regions of the green plants takes place due to
A)
indole acetic acid
done
clear
B)
cytokinins
done
clear
C)
gibberellins
done
clear
D)
ethylene
done
clear
View Answer play_arrow
question_answer 119) A nutritionally wild type organism, which does not require any additional growth supplement is known as
A)
phenotype
done
clear
B)
holotype
done
clear
C)
auxotroph
done
clear
D)
prototroph
done
clear
View Answer play_arrow
question_answer 120) Which of the following propagates through leaf-tip?
A)
Walking fem
done
clear
B)
Sprout-leaf plant
done
clear
C)
Marchantia
done
clear
D)
Moss
done
clear
View Answer play_arrow
question_answer 121) Common indicator organism of water pollution is
A)
Lemna pancicostata
done
clear
B)
Eichhomia crassipes
done
clear
C)
Escherichia coli
done
clear
D)
Entamoeba histolytica
done
clear
View Answer play_arrow
question_answer 122) According to Oparin, which one of the following was not present in the primitive atmosphere of the earth?
A)
Methane
done
clear
B)
Oxygen
done
clear
C)
Hydrogen
done
clear
D)
Water vapour
done
clear
View Answer play_arrow
question_answer 123) Which one of the following precedes re-formation of the nuclear envelope during M-phase of the cell cycle?
A)
Decondensation from chromosomes and reassembly of the nuclear lamina
done
clear
B)
Transcription from chromosomes and reassembly of the nuclear lamina
done
clear
C)
Formation of the contractile ring and formation of the phragmoplast
done
clear
D)
Formation of the contractile ring and transcription from chromosomes
done
clear
View Answer play_arrow
question_answer 124) In transgenics, expression of transgene in target tissue is determined by
A)
enhancer
done
clear
B)
transgene
done
clear
C)
promoter
done
clear
D)
reporter
done
clear
View Answer play_arrow
question_answer 125) One of the ex situ conservation method for endangered species is
A)
wild-life sanctuaries
done
clear
B)
biosphere reserves
done
clear
C)
cryopreservation
done
clear
D)
national parks
done
clear
View Answer play_arrow
question_answer 126) The Cri-du-chat syndrome is caused by change in chromosome structure involving
A)
deletion
done
clear
B)
duplication
done
clear
C)
inversion
done
clear
D)
translocation
done
clear
View Answer play_arrow
question_answer 127) When synapsis is complete all along the chromosome, the cell is said to have entered a stage called
A)
zygotene
done
clear
B)
pachytene
done
clear
C)
diplotene
done
clear
D)
diakinesis
done
clear
View Answer play_arrow
question_answer 128) How does pruning help in making the hedge dense?
A)
It induces the differentiation of new shoots from the rootstock
done
clear
B)
It frees axillary buds from apical dominance
done
clear
C)
The apical shoot grows faster after pruning
done
clear
D)
It releases wound hormones
done
clear
View Answer play_arrow
question_answer 129) Which one of the following statements is correct?
A)
Neurons regulate endocrine activity, but not vice versa
done
clear
B)
Endocrine glands regulate neural activity and nervous system regulates endocrine glands
done
clear
C)
Neither hormones control neural activity nor the neurons control endocrine activity
done
clear
D)
Endocrine glands regulate neural activity but not vice versa
done
clear
View Answer play_arrow
question_answer 130) Examination of blood of a person suspected of having anaemia, shows large, immature, nucleated erythrocytes without haemoglobin. Supplementing his diet with which of the following, is likely to alleviate his symptoms?
A)
Thiamine
done
clear
B)
Folic acid and cobalamine
done
clear
C)
Riboflavin
done
clear
D)
Iron compounds
done
clear
View Answer play_arrow
question_answer 131) In which of the following fruit the edible part is the aril?
A)
Apple
done
clear
B)
Pomegranate
done
clear
C)
Orange
done
clear
D)
Litchi
done
clear
View Answer play_arrow
question_answer 132) Which one of the following amino acid was not found to be synthesized in Millers experiment?
A)
Glycine
done
clear
B)
Aspartic acid
done
clear
C)
Glutamic acid
done
clear
D)
Alanine
done
clear
View Answer play_arrow
question_answer 133) Which one of the following is not used for construction of ecological pyramids?
A)
Dry weight
done
clear
B)
Number of individuals
done
clear
C)
Rate of energy flow
done
clear
D)
Fresh weight
done
clear
View Answer play_arrow
question_answer 134) Treatment of seed at low temperature under moist conditions to break its dormancy is called
A)
scarification
done
clear
B)
vernalization
done
clear
C)
chelation
done
clear
D)
stratification
done
clear
View Answer play_arrow
question_answer 135) Which one of the following is the most suitable, medium for culture of Drosophila melanogaster?
A)
Moist-bread
done
clear
B)
Agar agar
done
clear
C)
Ripe banana
done
clear
D)
Cow dung
done
clear
View Answer play_arrow
question_answer 136) The thalloid body of a slime mould (Myxomycetes) is known as
A)
protonema
done
clear
B)
Plasmodium
done
clear
C)
fruiting body
done
clear
D)
mycelium
done
clear
View Answer play_arrow
question_answer 137) In which mode of inheritance do you expect more maternal influence among the offspring?
A)
Autosomal
done
clear
B)
Cytoplasmic
done
clear
C)
Y-linked
done
clear
D)
X-linked
done
clear
View Answer play_arrow
question_answer 138) What type of placentation is seen in sweet pea?
A)
Basal
done
clear
B)
Axile
done
clear
C)
Free central
done
clear
D)
Marginal
done
clear
View Answer play_arrow
question_answer 139) An organic substance bound to an enzyme and essential for its activity is called
A)
coenzyme
done
clear
B)
holoenzyme
done
clear
C)
apoenzyme
done
clear
D)
isoenzyme
done
clear
View Answer play_arrow
question_answer 140) Evolutionary history of an organism is known as
A)
phylogeny
done
clear
B)
ancestry
done
clear
C)
paleontology
done
clear
D)
ontogeny
done
clear
View Answer play_arrow
question_answer 141) Sertoli cells are regulated by the pituitary hormone known as
A)
FSH
done
clear
B)
GH
done
clear
C)
prolactin
done
clear
D)
LH
done
clear
View Answer play_arrow
question_answer 142) Antiparallel strands of a DNA molecule means that
A)
one strand turns anti-clockwise
done
clear
B)
the phosphate groups of two DNA strands at their ends, share the same position
done
clear
C)
the phosphate groups at the start of two DNA strands are in opposite position (pole)
done
clear
D)
one strand turns clockwise
done
clear
View Answer play_arrow
question_answer 143) Restriction endonuclease
A)
cuts the DNA molecule randomly
done
clear
B)
cuts the DNA molecule at specific sites
done
clear
C)
restricts the synthesis of DNA inside the nucleus
done
clear
D)
synthesizes DNA
done
clear
View Answer play_arrow
question_answer 144) Earthworms are
A)
ureotelic when plenty of water is available
done
clear
B)
uricotelic when plenty of water is available
done
clear
C)
uricotelic under conditions of water scarcity
done
clear
D)
ammonotelic when plenty of water is available
done
clear
View Answer play_arrow
question_answer 145) Which one of the following has an open circulatory system?
A)
Pheretima
done
clear
B)
Periplaneta
done
clear
C)
Hirudinaria
done
clear
D)
Octopus
done
clear
View Answer play_arrow
question_answer 146) Biradial symmetry and lack of cnidoblasts are the characteristics of
A)
Starfish and sea anemone
done
clear
B)
Ctenoplana and Beroe
done
clear
C)
Aurelia and Paramecium
done
clear
D)
Hydra and starfish
done
clear
View Answer play_arrow
question_answer 147) In order to obtain virus- free plants through tissue culture, the best method is
A)
protoplast culture
done
clear
B)
embryo rescue
done
clear
C)
anther culture
done
clear
D)
meristem culture
done
clear
View Answer play_arrow
question_answer 148) Both sickle cell anaemia and Huntingtons chorea are
A)
bacteria-related diseases
done
clear
B)
congenital disorders
done
clear
C)
pollutant-induced disorders
done
clear
D)
virus-related diseases
done
clear
View Answer play_arrow
question_answer 149) Which one of the following pairs is not correctly matched?
A)
Vitamin \[{{B}_{12}}\] - Pernicious anaemia
done
clear
B)
Vitamin\[{{B}_{6}}\] - Loss of appetite
done
clear
C)
Vitamin\[{{B}_{1}}\] - Beri-beri
done
clear
D)
Vitamin\[{{B}_{2}}\] - Pellagra
done
clear
View Answer play_arrow
question_answer 150) Injury to vagus nerve in human is not likely to affect
A)
tongue movements
done
clear
B)
gastrointestinal movements
done
clear
C)
pancreatic secretion
done
clear
D)
cardiac movements
done
clear
View Answer play_arrow