A) \[C{{H}_{3}}+2{{O}_{2}}\xrightarrow{{}}C{{O}_{2}}+2{{H}_{2}}O\]
B) \[C{{H}_{4}}+4C{{l}_{2}}\xrightarrow{{}}CC{{l}_{4}}+4HCl\]
C) \[2{{F}_{2}}+2O{{H}^{-}}\xrightarrow{{}}2{{F}^{-}}+O{{F}_{2}}+{{H}_{2}}O\]
D) \[2N{{O}_{2}}+2O{{H}^{-}}\xrightarrow{{}}NO_{2}^{-}+NO_{3}^{-}+{{H}_{2}}O\]
Correct Answer: D
Solution :
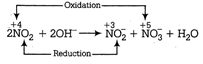 Since, in above reaction, the same element, i.e. N is reduced as well as oxidised, so it is a disproportionation reaction.
Since, in above reaction, the same element, i.e. N is reduced as well as oxidised, so it is a disproportionation reaction.
You need to login to perform this action.
You will be redirected in
3 sec
