A) 2-chlorobutane
B) 2, 3-dichlorobutane
C) 2, 3-dichloropentane
D) 2-hydroxypropanoic acid
Correct Answer: B
Solution :
[a]\[=C{{H}_{3}}-\underset{Cl}{\mathop{\underset{|}{\mathop{\overset{*}{\mathop{C}}\,}}\,}}\,H-C{{H}_{2}}C{{H}_{3}}\] One asymmetric carbon atom, forms d- and \[l-\]optical isomers.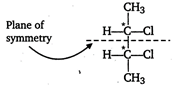 Meso due to internal compensation [b] Two asymmetric carbon atoms, forms \[d-,l-\]and meso forms. [c]\[C{{H}_{3}}-\underset{Cl}{\mathop{\underset{|}{\overset{*}{\mathop{CH}}}\,}}\,-\underset{Cl}{\mathop{\underset{|}{\overset{*}{\mathop{CH}}}\,}}\,-C{{H}_{2}}C{{H}_{3}}\] Two asymmetric carbon atoms but does not have symmetry. Hence, meso form is not formed. [d] \[C{{H}_{3}}-\underset{OH}{\mathop{\underset{|}{\overset{*}{\mathop{C}}}\,}}\,H-COOH\] One asymmetric carbon atom, meso form is not formed.
Meso due to internal compensation [b] Two asymmetric carbon atoms, forms \[d-,l-\]and meso forms. [c]\[C{{H}_{3}}-\underset{Cl}{\mathop{\underset{|}{\overset{*}{\mathop{CH}}}\,}}\,-\underset{Cl}{\mathop{\underset{|}{\overset{*}{\mathop{CH}}}\,}}\,-C{{H}_{2}}C{{H}_{3}}\] Two asymmetric carbon atoms but does not have symmetry. Hence, meso form is not formed. [d] \[C{{H}_{3}}-\underset{OH}{\mathop{\underset{|}{\overset{*}{\mathop{C}}}\,}}\,H-COOH\] One asymmetric carbon atom, meso form is not formed.
You need to login to perform this action.
You will be redirected in
3 sec
