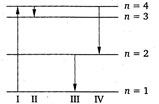
A) III
B) IV
C) I
D) II
Correct Answer: A
Solution :
\[E=Rhc\left[ \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right]\] \[{{E}_{(4\to 3)}}=Rhc\left[ \frac{1}{{{3}^{2}}}-\frac{1}{{{4}^{2}}} \right]\] \[=Rhc\left[ \frac{7}{9\times 16} \right]=0.05\,Rhc\] \[{{E}_{(4\to 2)}}=Rhc\left[ \frac{1}{{{2}^{2}}}-\frac{1}{{{4}^{2}}} \right]\] \[=Rhc\left[ \frac{3}{16} \right]=0.2\,Rhc\] \[{{E}_{(2\to 1)}}=Rhc\left[ \frac{1}{{{(1)}^{2}}}-\frac{1}{{{(2)}^{2}}} \right]\] \[=Rhc\left[ \frac{3}{4} \right]=0.75\,Rhc\] \[{{E}_{(1\to 3)}}=Rhc\left[ \frac{1}{{{(3)}^{2}}}-\frac{1}{{{(1)}^{2}}} \right]\] \[=-\frac{8}{9}Rhc=-0.9Rhc\] Thus, transition in gives most energy. Transition\[\text{I}\]represents the absorption of energy.You need to login to perform this action.
You will be redirected in
3 sec
