A) \[sp\]
B) \[s{{p}^{3}}\]
C) \[ds{{p}^{2}}\]
D) \[s{{p}^{2}}\]
Correct Answer: D
Solution :
S(excited state)=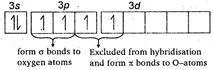 It has two bonds and one lone pair The\[S-O\]) double bond arise from \[p\pi -d\pi \]bonding due to lateral overlap of p-orbitals of oxygen with \[d-\]orbitals of sulphur. The bond between S and O results from \[s{{p}^{2}}\]hybridization in S-atom.
It has two bonds and one lone pair The\[S-O\]) double bond arise from \[p\pi -d\pi \]bonding due to lateral overlap of p-orbitals of oxygen with \[d-\]orbitals of sulphur. The bond between S and O results from \[s{{p}^{2}}\]hybridization in S-atom. 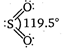
You need to login to perform this action.
You will be redirected in
3 sec
