A) \[11\sigma ,4\pi \]
B) \[12\sigma ,3\pi \]
C) \[14\sigma ,4\pi \]
D) \[15\sigma ,4\pi \]
Correct Answer: C
Solution :
Key Idea: First bond between any two atoms is sigma and rest are pi bonds.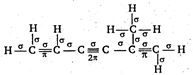 \[14\sigma \]and \[4\pi \]bonds are there.
\[14\sigma \]and \[4\pi \]bonds are there.
You need to login to perform this action.
You will be redirected in
3 sec
