A) \[{{H}_{2}}S\]
B) \[S{{O}_{2}}\]
C) \[S\]
D) \[{{H}_{2}}O\]
Correct Answer: A
Solution :
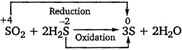 \[\therefore \]\[{{H}_{2}}S\]is oxidised in this reaction.
\[\therefore \]\[{{H}_{2}}S\]is oxidised in this reaction.
You need to login to perform this action.
You will be redirected in
3 sec
