A) \[6.2\times {{10}^{-11}}\]
B) \[16\times {{10}^{-9}}\]
C) \[16\times {{10}^{-11}}\]
D) \[16\times {{10}^{-13}}\]
Correct Answer: C
Solution :
Robert Millikan performed the experiment to determine the charge on an electron. When a drop is suspended, its weight mg is exactly equal to the electric force applied qE, where E is electric filed, q the charge, m the mass of drop and g the acceleration due to gravity.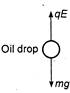 Hence, solving for q, we get \[q=\frac{mg}{E}\] Given,\[m=16\times {{10}^{-6}}kg,\text{ }g=10\text{ }m/{{s}^{2}},\] \[E={{10}^{6}}V/m\] Note: With the help of this experiment Millikan determined that there was a smallest unit charge or that charge is quantized. He received the noble prize for his work.
Hence, solving for q, we get \[q=\frac{mg}{E}\] Given,\[m=16\times {{10}^{-6}}kg,\text{ }g=10\text{ }m/{{s}^{2}},\] \[E={{10}^{6}}V/m\] Note: With the help of this experiment Millikan determined that there was a smallest unit charge or that charge is quantized. He received the noble prize for his work.
You need to login to perform this action.
You will be redirected in
3 sec
