question_answer 1) Which of the following is a true statement?
A)
The total entropy of thermally interacting systems is conserved
done
clear
B)
Carnot engine has 100% efficiency
done
clear
C)
Total entropy does not change in a reversible process
done
clear
D)
Total entropy in an irreversible process can either increase or decrease
done
clear
View Answer play_arrow
question_answer 2) Which of the following is not related to the Bernoullis principle?
A)
Rise of a liquid column inside a capillary
done
clear
B)
Operation of a venturimeter
done
clear
C)
Lift provided to an aeroplane by the air
done
clear
D)
Propelling force provided to an aeroplane by its propellers
done
clear
View Answer play_arrow
question_answer 3) A steel wire can support a maximum load of w before reaching its elastic limit. How much load can another wire, made out of identical steel, but with a radius one half the radius of the first wire, support before reaching its elastic limit?
A)
\[w\]
done
clear
B)
\[\frac{w}{2}\]
done
clear
C)
\[\frac{w}{4}\]
done
clear
D)
\[4w\]
done
clear
View Answer play_arrow
question_answer 4) Which of the following laws of thermodynamics forms the basis for the definition of temperature?
A)
First law
done
clear
B)
Zerothlaw
done
clear
C)
Second law
done
clear
D)
Third law
done
clear
View Answer play_arrow
question_answer 5) The air pressure inside a soap bubble of radius R exceeds the outside air pressure by 10 Pa. By how much will the pressure inside a bubble of radius 2R exceed the outside air pressure?
A)
20 Pa
done
clear
B)
40 Pa
done
clear
C)
2.5 Pa
done
clear
D)
5 Pa
done
clear
View Answer play_arrow
question_answer 6) Taking the radius of the earth to be 6400 km, by what percentage will the acceleration due to gravity at a height of 100 km from the surface of the earth differ from that on the surface of the earth?
A)
About 1.5%
done
clear
B)
About 5%
done
clear
C)
About 8%
done
clear
D)
About 3%
done
clear
View Answer play_arrow
question_answer 7) Which of the following statements is true for the three types of magnetism - para, dia and ferro?
A)
Paramagnetism is associated with negative susceptibility and dia and ferromagnetism with positive susceptibility
done
clear
B)
Diamagnetism is generally weakest of the three and is associated with negative susceptibility
done
clear
C)
Ferromagnetism is the strongest of the three and is associated with negative susceptibility
done
clear
D)
All three are associated with positive susceptibility, diamagnetism is the weakest form of magnetism and ferromagnetism is the strongest form
done
clear
View Answer play_arrow
question_answer 8) The molecules in an ideal gas at\[27{}^\circ C\]have a certain mean velocity. At what approximate temperature, will the mean velocity be doubled?
A)
\[54{}^\circ C\]
done
clear
B)
\[327{}^\circ C\]
done
clear
C)
\[1200{}^\circ C\]
done
clear
D)
\[927{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 9) Which of the two (i) compressing a gas isothermally until its volume is reduced by half (ii) compressing the same gas adiabatically until its volume is reduced by half, will require more work to be done?
A)
(i)
done
clear
B)
(ii)
done
clear
C)
Both will require the same amount of work
done
clear
D)
It will depend upon the nature of the gas
done
clear
View Answer play_arrow
question_answer 10) Three coplanar, parallel, long straight wires are equally spaced, that is, the distance between each pair of successive wires is the same. The first and the third wire carry currents of 1 A each, in the same direction. What must be the current in the second wire (wire in the middle), so that the other two wires do not feel any net force?
A)
0.25 A in opposite direction to those in the first and the third
done
clear
B)
0.5 A in the same direction as those in the first and the third
done
clear
C)
0.5 A in the opposite direction to those in the first and the third
done
clear
D)
0.25 A in the same direction as those in the first and the third
done
clear
View Answer play_arrow
question_answer 11) A conducting rod of length L is moving in a uniform magnetic field with a velocity v without rotation. The velocity of the rod is perpendicular to the rod, and the motion of the rod is confined to a plane perpendicular to the magnetic field. What is the induced emf developed across the rod?
A)
\[BLv\]
done
clear
B)
\[B{{v}^{2}}L\]
done
clear
C)
\[\frac{BL}{v}\]
done
clear
D)
\[B{{L}^{2}}v\]
done
clear
View Answer play_arrow
question_answer 12) What is the resonance frequency of a driven. L-C-R oscillator?
A)
\[\frac{1}{LC}\]
done
clear
B)
\[\frac{1}{2\pi LC}\]
done
clear
C)
\[{{(LC)}^{-1/2}}\]
done
clear
D)
\[{{(2\pi LC)}^{-1/2}}\]
done
clear
View Answer play_arrow
question_answer 13) A bar magnet is placed upright on a floor (so that the axis of the magnet is vertical). A copper ring is held above the magnet, with its plane horizontal and released. The copper ring falls in such a manner that its axis always coincides with that of/ the magnet. What will be the acceleration with which the ring will fall? Acceleration due to gravity is\[10\text{ }m/{{s}^{2}}\].
A)
\[10\text{ }m/{{s}^{2}}\]
done
clear
B)
less than\[10\text{ }m/{{s}^{2}}\]
done
clear
C)
more than\[10\text{ }m/{{s}^{2}}\]
done
clear
D)
the answer will depend upon which pole of the magnet is up
done
clear
View Answer play_arrow
question_answer 14) A short solenoid of radius a, number of turns per unit length n^ and length L is kept coaxially inside a very long solenoid of radius b, number of turns per unit length\[{{n}_{2}}\]. What is the mutual inductance of the system?
A)
\[{{\mu }_{0}}\pi {{b}^{2}}{{n}_{1}}{{n}_{2}}L\]
done
clear
B)
\[{{\mu }_{0}}\pi {{a}^{2}}{{n}_{1}}{{n}_{2}}{{L}^{2}}\]
done
clear
C)
\[{{\mu }_{0}}\pi {{a}^{2}}{{n}_{1}}{{n}_{2}}L\]
done
clear
D)
\[{{\mu }_{0}}\pi {{b}^{2}}{{n}_{1}}{{n}_{2}}{{L}^{2}}\]
done
clear
View Answer play_arrow
question_answer 15) Which of the following is a semiconductor?
A)
\[F{{e}_{2}}{{O}_{3}}\]
done
clear
B)
\[Si{{O}_{2}}\]
done
clear
C)
\[GaAs\]
done
clear
D)
\[CuO\]
done
clear
View Answer play_arrow
question_answer 16) What is the order of the reverse saturation current before break down in a Zener diode?
A)
Ampere
done
clear
B)
Milli-ampere
done
clear
C)
It depends on the applied voltage
done
clear
D)
Micro-ampere
done
clear
View Answer play_arrow
question_answer 17)
Which logic gate does the following truth table represent? Input A Input B Input Q 0 0 1 0 1 1 1 0 1 1 1 0
A)
NAND
done
clear
B)
AND
done
clear
C)
OR
done
clear
D)
NOR
done
clear
View Answer play_arrow
question_answer 18) What is the equivalent expression of the decimal number 212 in binary number system?
A)
11000100
done
clear
B)
10010100
done
clear
C)
11010100
done
clear
D)
11010110
done
clear
View Answer play_arrow
question_answer 19) Why do we need carrier wave of high frequency to transmit audio signal over long distances?
A)
High frequency carrier wave can propagate with a faster speed
done
clear
B)
High frequency carrier waves offer availability of higher transmission bandwidth
done
clear
C)
High frequency carrier waves offer availability of lower transmission bandwidth
done
clear
D)
High frequency carrier waves is easy to produce
done
clear
View Answer play_arrow
question_answer 20) What does the Poynting vector represent?
A)
Power flowing across unit area in an electromagnetic field
done
clear
B)
Charge flowing across unit area per unit time in an electromagnetic field
done
clear
C)
Momentum flowing across unit area per unit time in an electromagnetic field
done
clear
D)
Angular momentum flowing across unit area per unit time in an electromagnetic field
done
clear
View Answer play_arrow
question_answer 21) In television transmission what type of modulation is used?
A)
Only amplitude modulation
done
clear
B)
Only frequency modulation
done
clear
C)
Both amplitude and frequency modulation
done
clear
D)
TV signal does not need any kind of modulation
done
clear
View Answer play_arrow
question_answer 22) Which of the following is true for any collision?
A)
Both linear momentum and kinetic energy are conserved
done
clear
B)
Neither linear momentum nor kinetic energy may be conserved
done
clear
C)
Linear momentum is always conserved, however, kinetic energy may or may not be conserved
done
clear
D)
Kinetic energy is always conserved, but linear momentum may or may not be conserved
done
clear
View Answer play_arrow
question_answer 23) A uniform rod of length L and mass M is held vertical, with its bottom end pivoted to the floor. The rod falls under gravity, freely turning about the pivot. If acceleration due to gravity is g, what is the instantaneous angular speed of the rod when it makes an angle\[60{}^\circ \]with the vertical?
A)
\[{{\left( \frac{g}{L} \right)}^{1/2}}\]
done
clear
B)
\[{{\left( \frac{3g}{4L} \right)}^{1/2}}\]
done
clear
C)
\[{{\left( \frac{3\sqrt{3}g}{2L} \right)}^{1/2}}\]
done
clear
D)
\[{{\left( \frac{3g}{2L} \right)}^{1/2}}\]
done
clear
View Answer play_arrow
question_answer 24) A cheetah, weighing 150 kg, chases a deer, weighing 30 kg, in a straight path. The speed of the cheetah is 20 m/s and that of the deer is 25 m/s. The approximate speed of the centre of mass of the pair is
A)
21 m/s
done
clear
B)
24 m/s
done
clear
C)
26 m/s
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 25) A tennis racket can be idealized as a uniform ring of mass M and radius R, attached to a uniform rod also of mass M and length L. The rod and the ring are coplanar and the line of the rod passes through the centre of the ring. The moment of inertia of the object (racket) about an axis through the centre of the ring and perpendicular to its plane is
A)
\[\frac{1}{12}M(6{{R}^{2}}+{{L}^{2}})\]
done
clear
B)
\[\frac{1}{12}M(18{{R}^{2}}+{{L}^{2}})\]
done
clear
C)
\[\frac{1}{3}M(6{{R}^{2}}+{{L}^{2}}+3LR)\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 26) How long will a satellite, placed in a circular orbit of radius that is\[{{\left( \frac{1}{4} \right)}^{th}}\]the radius of a geostationary satellite, take to complete one revolution around the earth?
A)
12 h
done
clear
B)
6 h
done
clear
C)
3 h
done
clear
D)
4 days
done
clear
View Answer play_arrow
question_answer 27) A rocket is fired from inside a deep mine, so as to escape the earths gravitational field. The minimum velocity to be rocket is
A)
exactly the same as the escape velocity of fire from the earths surface
done
clear
B)
a little more than the escape velocity of fire from the earths surface
done
clear
C)
a little less than the escape velocity of fire from the earths surface
done
clear
D)
Infinity
done
clear
View Answer play_arrow
question_answer 28) Which of the following is the correct Kirchhoffs loop rule?
A)
The algebraic sum of the currents meeting at a junction is zero
done
clear
B)
The algebraic sum of potential drops, across all resistors in a circuit is zero
done
clear
C)
The algebraic sum of the currents across all the resistors in a circuit is zero
done
clear
D)
The algebraic sum of potential drops across all resistors plus those across sources in a circuit is zero
done
clear
View Answer play_arrow
question_answer 29) What are the dimensions of electrical conductivity?
A)
\[[M{{L}^{-3}}{{T}^{3}}{{I}^{2}}]\]
done
clear
B)
\[[{{M}^{-1}}{{L}^{3}}{{T}^{2}}{{I}^{3}}]\]
done
clear
C)
\[[{{M}^{-1}}{{L}^{-2}}{{T}^{3}}{{I}^{2}}]\]
done
clear
D)
\[[{{M}^{-1}}{{L}^{-3}}{{T}^{3}}I]\]
done
clear
View Answer play_arrow
question_answer 30) A coil has resistance\[25.00\,\Omega \]and\[25.17\,\Omega \]at \[20{}^\circ C\]and\[35{}^\circ C\]respectively. What is the temperature coefficient of resistance?
A)
\[4.545\times {{10}^{-7}}/{}^\circ C\]
done
clear
B)
\[4.545\times {{10}^{-3}}/{}^\circ C\]
done
clear
C)
\[4.545\times {{10}^{-2}}/{}^\circ C\]
done
clear
D)
\[4.545\times {{10}^{-5}}/{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 31) An electron and a proton, both having the same kinetic energy, enter a region of uniform magnetic field, in a plane perpendicular to the field. If their masses are denoted by\[{{m}_{e}}\]and\[{{m}_{p}}\] respectively, then the ratio of the radii (electron to proton) of their circular orbits is
A)
\[\sqrt{\frac{{{m}_{p}}}{{{m}_{e}}}}\]
done
clear
B)
\[\sqrt{\frac{{{m}_{e}}}{{{m}_{p}}}}\]
done
clear
C)
\[\frac{{{m}_{e}}}{{{m}_{p}}}\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 32) In using Amperes law to find the magnetic field of a straight, long solenoid, the loop (Amperian loop) that is taken is
A)
a circular loop, coaxial with the solenoid
done
clear
B)
a rectangular loop in a plane perpendicular to the axis of the solenoid
done
clear
C)
a rectangular loop in a plane containing the axis of the solenoid, the loop being totally within the solenoid
done
clear
D)
a rectangular loop in a plane containing the axis of the solenoid, the loop being partly inside the solenoid and partly outside it
done
clear
View Answer play_arrow
question_answer 33) A rectangular coil, of sides 2 cm and 3 cm respectively, has 10 turns in it. It carries a current of 1 A, and is placed in a uniform magnetic field of 0.2 T in such a manner that its plane makes an angle\[60{}^\circ \]with the field direction. The torque on the loop is
A)
\[6.0\times {{10}^{-4}}N-m\]
done
clear
B)
\[6.0\times {{10}^{-5}}N-m\]
done
clear
C)
\[1.2\times {{10}^{-3}}N-m\]
done
clear
D)
6.0 N-m
done
clear
View Answer play_arrow
question_answer 34) Which of the following facts about the photoelectric effect can be understood without invoking the quantum concept of light propagation?
A)
The rate of photoelectrons emission, when they are emitted, increases with the intensity of light used
done
clear
B)
There is a threshold frequency, below which no photoelectrons are emitted, no matter how long the light is thrown on the metallic surface
done
clear
C)
Once the frequency of light is more than the threshold frequency, photoelectrons are emitted almost instantaneously, no matter how weak the light intensity is
done
clear
D)
For each frequency of light, exceeding the threshold frequency, there is a maximum kinetic energy of the emitted electrons
done
clear
View Answer play_arrow
question_answer 35) Consider the four gases-hydrogen, oxygen, nitrogen and helium, at the same temperature. Arrange them in the increasing order of the de-Broglie wavelengths of their molecules
A)
hydrogen, helium, nitrogen, oxygen
done
clear
B)
oxygen, nitrogen, hydrogen, helium
done
clear
C)
oxygen, nitrogen, helium, hydrogen
done
clear
D)
nitrogen, oxygen, helium, hydrogen
done
clear
View Answer play_arrow
question_answer 36) The half-life of\[^{60}Co\] is approximately 5.25 years. In a sample containing 1 g of freshly prepared\[^{60}Co,\]how much of the isotope will be left after 21 years?
A)
125 mg
done
clear
B)
62.5 mg
done
clear
C)
Nothing will be left
done
clear
D)
31.25 mg
done
clear
View Answer play_arrow
question_answer 37) Which of the following is true of the Balmer series of the hydrogen spectrum?
A)
The entire series falls in the ultraviolet region
done
clear
B)
The entire series falls in the infrared region
done
clear
C)
The series is partly in the visible region and partly in the ultraviolet region
done
clear
D)
The series is partly in the visible region and partly in the infrared region
done
clear
View Answer play_arrow
question_answer 38) In a nuclear fusion reaction, two nuclei, A and B, fuse to produce a nucleus C, releasing an amount of energy\[\Delta E\]in the process. If the mass defects of the three nuclei are\[\Delta {{M}_{A}},\Delta {{M}_{B}}\]and\[\Delta {{M}_{C}}\]respectively, then which of the following relations holds? Here, c is the speed of light
A)
\[\Delta {{M}_{A}}+\Delta {{M}_{B}}=\Delta {{M}_{C}}-\Delta E/{{c}^{2}}\]
done
clear
B)
\[\Delta {{M}_{A}}+\Delta {{M}_{B}}=\Delta {{M}_{C}}+\Delta E/{{c}^{2}}\]
done
clear
C)
\[\Delta {{M}_{A}}-\Delta {{M}_{B}}=\Delta {{M}_{C}}-\Delta E/{{c}^{2}}\]
done
clear
D)
\[\Delta {{M}_{A}}-\Delta {{M}_{B}}=\Delta {{M}_{C}}+\Delta E/{{c}^{2}}\]
done
clear
View Answer play_arrow
question_answer 39) Which of the following postulates of the Bohr model led to the quantization of energy of the hydrogen atom?
A)
The electron goes around the nucleus in circular orbits
done
clear
B)
The angular momentum of the electron can only be an integral multiple of h/2n
done
clear
C)
The magnitude of the linear momentum of the electron is quantized
done
clear
D)
Quantization of energy is itself a postulate of the Bohr model
done
clear
View Answer play_arrow
question_answer 40) A certain vector in the\[x-y\]plane has an \[x-\] component of 12 m and a\[y-\]component of 8 m. It is then rotated in the\[x-y\]plane so that its\[x-\]component is halved. Then its new y-component is approximately
A)
14m
done
clear
B)
13.11m
done
clear
C)
10m
done
clear
D)
2.0m
done
clear
View Answer play_arrow
question_answer 41) A block is placed on a plane inclined at\[12{}^\circ \]to the horizontal. What is the, maximum value of coefficient of static friction for which the block slides down the plane?
A)
\[tan\text{ }12{}^\circ \]
done
clear
B)
\[\text{cos }12{}^\circ \]
done
clear
C)
\[\text{sin }12{}^\circ \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 42) A brick of mass m , tied to a rope, is being whirled in a vertical circle, with a uniform speed. The tension in the rope is
A)
the same throughout
done
clear
B)
largest when the brick is at the highest point of the circular path and smallest when it is at the lowest point
done
clear
C)
largest when the rope is horizontal and smallest when it is vertical
done
clear
D)
largest when the brick is at the lowest point and smallest when it is at the highest point
done
clear
View Answer play_arrow
question_answer 43) Two blocks, of mass 1 kg and 2 kg respectively, are connected by a spring and kept on a frictionless table. The blocks are pulled apart, so that the spring is stretched, and released from rest. At a certain instant of time, the block of mass 1 kg, is found to be moving at a speed 2 m/s. What must be the speed of the other block at this instant?
A)
1 m/s
done
clear
B)
0.5 m/s
done
clear
C)
4 m/s
done
clear
D)
0.25 m/s
done
clear
View Answer play_arrow
question_answer 44) A coin of mass 10 g rolls along a horizontal table with a velocity of 6 cm/s. Its total kinetic energy is
A)
\[9\,\mu J\]
done
clear
B)
\[18\,\mu J\]
done
clear
C)
\[27\,\mu J\]
done
clear
D)
\[36\,\mu J\]
done
clear
View Answer play_arrow
question_answer 45) A simple harmonic oscillator oscillates, with an amplitude A. At what point of its motion, is the power delivered to it by the restoring force maximum?
A)
When it is at a displacement\[\pm \frac{A}{\sqrt{2}}\]from the equilibrium point and moving towards the equilibrium point
done
clear
B)
When it is at the maximum displacement
done
clear
C)
When it passes through the equilibrium point, either way
done
clear
D)
When it is at a displacement\[\pm \frac{A}{\sqrt{2}}\]from the equilibrium point and moving away from the equilibrium point
done
clear
View Answer play_arrow
question_answer 46) There is a point charge q located at the centre of a cube. What is the electric flux of this point charge, through a face of the cube?
A)
\[\frac{q}{{{\varepsilon }_{0}}}\]
done
clear
B)
\[\frac{q}{6{{\varepsilon }_{0}}}\]
done
clear
C)
\[\frac{q}{3{{\varepsilon }_{0}}}\]
done
clear
D)
It will depend upon the size of the cube
done
clear
View Answer play_arrow
question_answer 47) A point dipole is located at the origin in some orientation. The electric field at the point (10 cm, 10 cm) on the\[x-y\]plane is measured to have a magnitude\[1.0\times {{10}^{-3}}V/m\]. What will be the magnitude of the electric field at the point (20 cm, 20 cm)?
A)
\[5.0\times {{10}^{-4}}V/m\]
done
clear
B)
\[2.5\times {{10}^{-4}}V/m\]
done
clear
C)
It will depend on the orientation of the dipole
done
clear
D)
\[1.25\times {{10}^{-4}}V/m\]
done
clear
View Answer play_arrow
question_answer 48) Which of the following statements is false for a perfect conductor?
A)
The surface of the conductor is an equipotential surface
done
clear
B)
The electric field just outside the surface of a conductor is perpendicular to the surface
done
clear
C)
The charge carried by a conductor is always uniformly distributed over the surface of the conductor
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 49) A parallel plate capacitor without any dielectric within its plates, has a capacitance C and is connected to a battery of emf V. The battery is disconnected and the plates of the capacitor are pulled apart until the separation between the plates is doubled. What is the work done by the agent pulling the plates apart, in this process?
A)
\[\frac{1}{2}C{{V}^{2}}\]
done
clear
B)
\[\frac{3}{2}C{{V}^{2}}\]
done
clear
C)
\[-\frac{3}{2}C{{V}^{2}}\]
done
clear
D)
\[C{{V}^{2}}\]
done
clear
View Answer play_arrow
question_answer 50) Consider a copper wire of length L, cross-sectional area A. It has n number of free electrons per unit volume. Which of the following is the correct expression of drift velocity of the electrons when the wire carries a steady current\[I\]?
A)
\[\frac{I}{neL}\]
done
clear
B)
\[\frac{I}{{{n}^{2}}eL}\]
done
clear
C)
\[\frac{I}{neL}\]
done
clear
D)
\[\frac{I}{n{{e}^{2}}L}\]
done
clear
View Answer play_arrow
question_answer 51) A resistor has the following colour code, sequentially from the left Black Brown Orange Red and Black What is the resistance of the resistor?
A)
\[13\,\Omega \]
done
clear
B)
\[1300\,\,\Omega \]
done
clear
C)
\[130\,\,\Omega \]
done
clear
D)
\[13000\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 52) Which of the following is false for interference of light?
A)
Coherence of the sources is an essential condition for interference
done
clear
B)
The minima of the interference pattern need not be of zero intensity
done
clear
C)
Interference simply redistributes light energy, without destroying any of it
done
clear
D)
The minima of the interference pattern must always be of zero intensity
done
clear
View Answer play_arrow
question_answer 53) Totally unpolarized light of intensity\[{{I}_{0}}\]is incident normally on a polarizer and the emerging light is made to pass through a second, parallel polarizer with its axis making an angle of\[60{}^\circ \]with that of the first. What is the intensity of light emerging out of the second polarizer?
A)
Zero
done
clear
B)
\[\frac{{{I}_{0}}}{8}\]
done
clear
C)
\[\frac{{{I}_{0}}}{4}\]
done
clear
D)
\[\frac{{{I}_{0}}}{16}\]
done
clear
View Answer play_arrow
question_answer 54) A concave mirror has a focal length of 5 cm. When an object is placed at a distance of 15 cm from the mirror, where is the image formed?
A)
10 cm in front of the mirror
done
clear
B)
7.5 cm behind the mirror
done
clear
C)
2.5 cm in front of the mirror
done
clear
D)
7.5 cm in front of the mirror
done
clear
View Answer play_arrow
question_answer 55) The power of a convex lens is 2 dioptre. Its power is to be reduced to 1.5 dioptre, by putting another lens in combination with it. Which of the following lenses will serve the purpose?
A)
A concave lens of focal length 2 m
done
clear
B)
A concave lens of focal lens 4 m
done
clear
C)
A convex lens of focal lens 2 m
done
clear
D)
A concave lens of focal length 1 m
done
clear
View Answer play_arrow
question_answer 56) Spherical wave fronts, emanating from a point source, strike a plane reflecting surface. What will happen to these wave fronts, immediately after reflection?
A)
They will remain spherical with the same curvature, both in magnitude and sign
done
clear
B)
They will become plane wave fronts
done
clear
C)
They will remain spherical, with the same curvature, but sign of curvature reversed
done
clear
D)
They will remain spherical, but with different curvature, both in magnitude and sign
done
clear
View Answer play_arrow
question_answer 57) Which of the following is true for the minimum angular separation of two stars,\[\Delta {{\theta }_{\min }}\]at can be resolved by a telescope? In the following aperture is the diameter of the objective
A)
it decreases with the increase in aperture of the telescope
done
clear
B)
it is independent of the aperture of the telescope
done
clear
C)
it increases linearly with the aperture of the telescope
done
clear
D)
it increases quadratically with the aperture of the telescope
done
clear
View Answer play_arrow
question_answer 58) The flux density of mass is defined as the amount of mass crossing unit area per unit time. The dimensions of this quantity is
A)
\[[M{{L}^{-2}}{{T}^{-1}}]\]
done
clear
B)
\[[M{{L}^{2}}{{T}^{-1}}]\]
done
clear
C)
\[[ML{{T}^{-1}}]\]
done
clear
D)
\[[{{M}^{-1}}{{L}^{-2}}T]\]
done
clear
View Answer play_arrow
question_answer 59) A physical quantity z, depends upon two other physical quantities\[x\]and y, as follows. \[z=a{{x}^{2}}{{y}^{1/2}}\]where, a is a constant. In an experiment, the quantity\[x\]is determined by measuring z and y and using the above expression. If the percentage of error in the measurement of z and y are 10% and 12% respectively, then the percentage of error in the determined value of\[x\]is
A)
2%
done
clear
B)
8%
done
clear
C)
15%
done
clear
D)
without the value of the constant a, the percentage of error cannot be calculated
done
clear
View Answer play_arrow
question_answer 60) If a particle moves with an acceleration, then which of the following can remain constant?
A)
Both speed and velocity
done
clear
B)
Neither speed nor velocity
done
clear
C)
Only the velocity
done
clear
D)
Only the speed
done
clear
View Answer play_arrow
question_answer 61) A rubber ball is bounced on the floor of a room which has its ceiling at a height of 3.2 m from the floor. The ball hits the floor with a speed of 10 m/s and rebounds vertically up. If all collisions simply reverse the velocity of the ball, without changing its speed, then how long does it take the ball for a round trip, from the moment it bounces from the floor to the moment it returns back to it? Acceleration due to gravity is\[10\text{ }m/{{s}^{2}}\].
A)
4s
done
clear
B)
2s
done
clear
C)
0.8 s
done
clear
D)
1.2 s
done
clear
View Answer play_arrow
question_answer 62) Two vectors a and b, add up to vector c. When vector a is made 3 times as long and vector b is doubled in length, without changing their directions, then it is found that vector c is also doubled in length, without change in direction. Then which of the following is true?
A)
All three vectors must be parallel
done
clear
B)
b and c must be parallel to each other, but a need not be parallel to b and c
done
clear
C)
a and b must be perpendicular to each other
done
clear
D)
It is impossible for three non-zero vectors a, b and c to have the property stated above
done
clear
View Answer play_arrow
question_answer 63) Which of the following is not an assumption of the kinetic theory of gases?
A)
The molecules travel in straight paths until they undergo collision with other molecules
done
clear
B)
Molecules of the gas are small hard spheres, occupying negligible volume compared with the total volume of the gas
done
clear
C)
The molecules do not undergo any collisions at all
done
clear
D)
The molecules undergo elastic collisions only
done
clear
View Answer play_arrow
question_answer 64) The equation describing the motion of a simple harmonic oscillator along the\[x\]axis is given as \[x=A\cos (\omega t+\phi )\]. If at time\[t=0,\]the oscillator is at\[x=0\]and moving in the negative\[x-\]direction, then the phase angle\[\phi \]is
A)
\[\frac{\pi }{2}\]
done
clear
B)
\[-\frac{\pi }{2}\]
done
clear
C)
\[\pi \]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 65) At a displacement from the equilibrium position, that is one-half the amplitude of oscillation, what fraction of the total energy of the oscillator is kinetic energy?
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{4}\]
done
clear
C)
\[\frac{1}{\sqrt{2}}\]
done
clear
D)
\[\frac{3}{4}\]
done
clear
View Answer play_arrow
question_answer 66) Which of the following does not change as a wave moves from one medium to another?
A)
Wavelength
done
clear
B)
Wave velocity
done
clear
C)
Frequency
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 67) An organ pipe is closed at one end and open at the other. What is the ratio of frequencies of the 3rd and 4th fundamental modes of vibration?
A)
\[\frac{3}{4}\]
done
clear
B)
\[\frac{5}{7}\]
done
clear
C)
\[\frac{3}{5}\]
done
clear
D)
\[\frac{9}{11}\]
done
clear
View Answer play_arrow
question_answer 68) The Doppler shift in the frequency received by a stationary receiver when the source is moving towards it, was measured to be\[\Delta {{v}_{air}}\]when both receiver and source are in air, and it was measured to be\[\Delta {{v}_{water}}\]when both are under water. Then,
A)
\[\Delta {{v}_{air}}>\Delta {{v}_{water}}\]
done
clear
B)
\[\Delta {{v}_{air}}<\Delta {{v}_{water}}\]
done
clear
C)
\[\Delta {{v}_{air}}=\Delta {{v}_{water}}\]
done
clear
D)
\[\Delta {{v}_{water}}=0,\Delta {{v}_{air}}<0\]
done
clear
View Answer play_arrow
question_answer 69) Which of the following is false for electric lines of force?
A)
They always start from positive charges and terminate on negative charges
done
clear
B)
They are always perpendicular to the surface of a charged conductor
done
clear
C)
They always form closed loops
done
clear
D)
They are parallel and equally spaced in a region of uniform electric field
done
clear
View Answer play_arrow
question_answer 70) A uniform magnetic field B points vertically up and is slowly changed in magnitude, but not in direction. The rate of change of the magnetic field is a. A conducting ring of radius r and resistance R is held perpendicular to the magnetic field, and is totally inside it. The induced current in the ring is
A)
zero
done
clear
B)
\[\frac{2\pi rB}{R}\]
done
clear
C)
\[\frac{r\alpha }{R}\]
done
clear
D)
\[\frac{\pi {{r}^{2}}\alpha }{R}\]
done
clear
View Answer play_arrow
question_answer 71) A plane electromagnetic wave is propagating along the z-direction. If the electric field component of this wave is in the direction \[(i+j),\]then which of the following, is the direction of the magnetic field component?
A)
\[(-i+j)\]
done
clear
B)
\[(i-j)\]
done
clear
C)
\[(-i-j)\]
done
clear
D)
\[(i+j)\]
done
clear
View Answer play_arrow
question_answer 72) Which of the following is the correct arrangement of the electromagnetic spectrum in the increasing order of frequency?
A)
Microwaves, infrared, radio waves, visible light. X-rays
done
clear
B)
Radio waves, microwaves, infrared, visible light. X-rays
done
clear
C)
X-rays, visible light, infrared, microwaves, radio waves
done
clear
D)
Microwaves, radio waves, infrared, visible light. X-rays
done
clear
View Answer play_arrow
question_answer 73) Which of the following optical phenomena is involved in the propagation of light in an optical fibre?
A)
Refraction
done
clear
B)
Dispersion
done
clear
C)
Interference
done
clear
D)
Total internal reflection
done
clear
View Answer play_arrow
question_answer 74) In a Youngs double-slits experimental arrangement, the light used has wavelength \[5000\text{ }\overset{o}{\mathop{\text{A}}}\,,\] the slit separation is 2 mm and slits to screen distance is 1 m. What is the width of the fringes produced on the screen?
A)
0.25 mm
done
clear
B)
0.1 mm
done
clear
C)
0.5 mm
done
clear
D)
0.025 mm
done
clear
View Answer play_arrow
question_answer 75) In a single-slit diffraction experiment, the width of the slit is reduced by half. Which of the following needs to be done if the width of the central maxima has to remain the same?
A)
Reduce the distance between the slit and screen by half
done
clear
B)
Reduce the distance between the slit and the screen to\[{{\left( \frac{1}{4} \right)}^{th}}\]the original separation
done
clear
C)
Double the distance between the slit and the screen
done
clear
D)
No need to do anything, as the width of the central maxima does not depend on the slit width
done
clear
View Answer play_arrow
question_answer 76) Molar conductivity decreases with decrease in concentration
A)
for strong electrolytes
done
clear
B)
for weak electrolytes
done
clear
C)
both for strong and weak electrolytes
done
clear
D)
for non-electrolytes
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
question_answer 77) A nucleophilic substitution reaction proceeds through\[{{S}_{N}}1\]mechanism. So, the reaction is
A)
unimolecular
done
clear
B)
bimolecular
done
clear
C)
trimolecular
done
clear
D)
rate depends on concentration of incoming nucleophile
done
clear
View Answer play_arrow
question_answer 78) For an electrophilic aromatic substitution reaction
A)
chlorine is o-p directing group and also electron releasing group
done
clear
B)
chlorine is o-p directing group and also electron with drawing group
done
clear
C)
chlorine is meta directing group and also electron relasing group
done
clear
D)
chlorine is meta directing group and also electron withdrawing group
done
clear
View Answer play_arrow
question_answer 79) Enthalpy of combustion of carbon to\[C{{O}_{2}}\]is\[-393.52\text{ }kJ/mol\]. The heat released upon formation of 11 g of\[C{{O}_{2}}\]from carbon and dioxygen is
A)
35.77 kJ
done
clear
B)
98.38 kJ
done
clear
C)
1574.08 kJ
done
clear
D)
393.52 kJ
done
clear
View Answer play_arrow
question_answer 80) Entropy change in a process where 1 L of liquid He is poured into ice cold water is
A)
finite and positive
done
clear
B)
finite and negative
done
clear
C)
zero
done
clear
D)
infinity
done
clear
View Answer play_arrow
question_answer 81) Anode reaction of a fuel cell is
A)
\[Zn(Hg)+2O{{H}^{-}}\xrightarrow[{}]{{}}ZnO(s)+{{H}_{2}}O+2{{e}^{-}}\]
done
clear
B)
\[Pb(s)+SO_{4}^{2-}(aq)\xrightarrow[{}]{{}}PbS{{O}_{4}}(s)+2{{e}^{-}}\]
done
clear
C)
\[2{{H}_{2}}(g)+4O{{H}^{-}}(aq)\xrightarrow[{}]{{}}4{{H}_{2}}O(l)+4{{e}^{-}}\]
done
clear
D)
\[2Fe(s)\xrightarrow[{}]{{}}2F{{e}^{2+}}+4{{e}^{-}}\]
done
clear
View Answer play_arrow
question_answer 82) For an ideal system at thermal equilibrium, the velocity distribution of the constituent particles will be governed by
A)
Gaussian distribution
done
clear
B)
Maxwell-Boltzmann distribution
done
clear
C)
Lorentzian distribution
done
clear
D)
Log-normal distribution
done
clear
View Answer play_arrow
question_answer 83) During spontaneous discharge of an electrochemical cell Gibbs free energy will
A)
increase
done
clear
B)
decrease
done
clear
C)
not change
done
clear
D)
be infinity
done
clear
View Answer play_arrow
question_answer 84) Standard electrode potential of half-cell reactions are given below \[C{{u}^{2+}}+2{{e}^{-}}\xrightarrow[{}]{{}}Cu;\] \[E{}^\circ =0.34V\] \[Z{{n}^{2+}}+2{{e}^{-}}\xrightarrow[{}]{{}}Zn;\] \[E{}^\circ =-0.76V\] What is the emf of the cell?
A)
\[+1.10V\]
done
clear
B)
\[-1.10V\]
done
clear
C)
\[-0.42V\]
done
clear
D)
\[+0.42V\]
done
clear
View Answer play_arrow
question_answer 85) Tollens test can be used to distinguish
A)
propionaldehyde and acetone
done
clear
B)
propanol and propionic acid
done
clear
C)
propene and isobutene
done
clear
D)
isopropanol and propanol
done
clear
View Answer play_arrow
question_answer 86) The product of reaction between aniline and acetic anhydride is
A)
o-aminoacetophenone
done
clear
B)
m-aminoacetophenone
done
clear
C)
p-aminoacetophenone
done
clear
D)
acetanilide
done
clear
View Answer play_arrow
question_answer 87) According to Le-Chateliers principle, the equilibrium constant of a reversible reaction will not shift by
A)
increasing the temperature of an exothermic reaction
done
clear
B)
increasing the temperature of an endothermic reaction
done
clear
C)
changing the concentrations of the reactants
done
clear
D)
the effect of a catalyst
done
clear
View Answer play_arrow
question_answer 88) Properties of elements are periodic function of number of...... present in the nucleus.
A)
protons
done
clear
B)
electrons
done
clear
C)
neutrons
done
clear
D)
mesons
done
clear
View Answer play_arrow
question_answer 89) The complex\[{{[Co{{(N{{H}_{3}})}_{5}}Br]}^{2+}}SO_{4}^{2-}\]and\[{{[Co{{(N{{H}_{3}})}_{5}}S{{O}_{4}}]}^{+}}B{{r}^{-}}\]are
A)
coordination isomers
done
clear
B)
linkage isomers
done
clear
C)
stereoisomers
done
clear
D)
ionisation isomers
done
clear
View Answer play_arrow
question_answer 90) Maximum number of electrons in a shell with principal quantum number n is given by
A)
\[n\]
done
clear
B)
\[2n\]
done
clear
C)
\[{{n}^{2}}\]
done
clear
D)
\[2{{n}^{2}}\]
done
clear
View Answer play_arrow
question_answer 91) Anti-Markownikoff addition of\[HBr\]is not observed in
A)
propene
done
clear
B)
butene
done
clear
C)
2-butene
done
clear
D)
2-pentene
done
clear
View Answer play_arrow
question_answer 92) A ketone gives a yellow ppt when treated with 12 in an alkaline solution. Thus, the ketone is
A)
a cyclic ketone
done
clear
B)
a methyl ketone
done
clear
C)
an unsaturated ketone
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 93) Wroughtiron contains
A)
Cr
done
clear
B)
Cu
done
clear
C)
C
done
clear
D)
Ag
done
clear
View Answer play_arrow
question_answer 94) The ore magnetite is
A)
\[F{{e}_{3}}{{O}_{4}}\]
done
clear
B)
\[ZnC{{O}_{3}}\]
done
clear
C)
\[CuC{{O}_{3}}-Cu{{(OH)}_{2}}\]
done
clear
D)
\[Fe{{S}_{2}}\]
done
clear
View Answer play_arrow
question_answer 95) The first step in the extraction of Cu from copper pyrites is
A)
reduction by carbon
done
clear
B)
electrolysis of ore
done
clear
C)
roasting of ore in\[{{O}_{2}}\]
done
clear
D)
magnetic separation
done
clear
View Answer play_arrow
question_answer 96) Desalination of sea water can be done by
A)
osmosis
done
clear
B)
reverse osmosis
done
clear
C)
filtration
done
clear
D)
diffusion
done
clear
View Answer play_arrow
question_answer 97) A compound with nitro group was reduced by \[Sn/HCl,\]followed by treatment with \[NaN{{O}_{2}}/HCl\]and followed by phenol. The chromophore group in the final compound is
A)
\[N{{O}_{2}}\]group
done
clear
B)
\[N{{H}_{2}}\]group
done
clear
C)
azo group
done
clear
D)
OH group
done
clear
View Answer play_arrow
question_answer 98)
Certain reactions follow the relation between concentrations of the reactant vs time as
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
Infinity
done
clear
View Answer play_arrow
question_answer 99) A first order reaction has a rate constant\[k=3.01\times {{10}^{-3}}/s\]. How long it will take to decompose half of the reaction?
A)
2.303 s
done
clear
B)
23.03 s
done
clear
C)
230.23 s
done
clear
D)
2303 s
done
clear
View Answer play_arrow
question_answer 100) The shape of the ammonia molecule is
A)
tetrahedral
done
clear
B)
trigonal pyramidal
done
clear
C)
trigonal bipyramid
done
clear
D)
trigonal planar
done
clear
View Answer play_arrow
question_answer 101) \[{{H}_{5}}I{{O}_{6}}\]is a
A)
strong reducing agent
done
clear
B)
strong base
done
clear
C)
strong oxidising agent
done
clear
D)
weak base
done
clear
View Answer play_arrow
question_answer 102) If a compound gives an orange or red precipitate with 2,4-dinitrophenylhydrazine, then the compound is
A)
an alkyl halide
done
clear
B)
an aryl halide
done
clear
C)
an amine
done
clear
D)
a carbonyl compound
done
clear
View Answer play_arrow
question_answer 103) Isotones have
A)
same neutron number but different proton number
done
clear
B)
same proton number but different neutron number
done
clear
C)
same proton and neutron number
done
clear
D)
same proton but different electron number
done
clear
View Answer play_arrow
question_answer 104) \[{{(C{{H}_{3}})}_{3}}C-OH\]on treatment with\[NaCl\]in aqueous medium gives
A)
no reaction
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}{{C}^{-}}N{{a}^{+}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{3}}CC{{l}^{-}}\]
done
clear
D)
isobutylene
done
clear
View Answer play_arrow
question_answer 105) The de-Broglie wavelength of a particle is
A)
proportional to its mass
done
clear
B)
proportional to its velocity
done
clear
C)
inversely proportional to its momentum
done
clear
D)
proportional to its total energy
done
clear
View Answer play_arrow
question_answer 106) The\[C-H\]bond distance is the longest in
A)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
C)
\[{{C}_{2}}He\]
done
clear
D)
\[{{C}_{2}}{{H}_{2}}B{{r}_{2}}\]
done
clear
View Answer play_arrow
question_answer 107) Dimension of universal gas constant (R) is
A)
\[[VP{{T}^{-1}}{{n}^{-1}}]\]
done
clear
B)
\[[V{{P}^{-1}}T{{n}^{-1}}]\]
done
clear
C)
\[[VPT{{n}^{-1}}]\]
done
clear
D)
\[[VP{{T}^{-1}}n]\]
done
clear
View Answer play_arrow
question_answer 108) Entropy of a perfectly crystalline solid at\[0\text{ }K\]is
A)
positive
done
clear
B)
negative
done
clear
C)
zero
done
clear
D)
either positive or negative
done
clear
View Answer play_arrow
question_answer 109) The hybridisation of carbon in molecular\[C{{O}_{2}}\] is
A)
\[sp\]
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{3}}\]
done
clear
D)
\[s{{p}^{3}}d\]
done
clear
View Answer play_arrow
question_answer 110) \[CC{{l}_{4}}\]and freons
A)
are green compounds because they are green coloured
done
clear
B)
deplete ozone
done
clear
C)
cause increase in ozone concentration
done
clear
D)
have no effect on ozone concentration
done
clear
View Answer play_arrow
question_answer 111) Total number of metal atoms per unit cell in a face-centred cubic lattice is
A)
14
done
clear
B)
8
done
clear
C)
6
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 112) Bakelite is formed by polymerization between
A)
acrylonitrile molecules
done
clear
B)
tetrafluoroethene molecules
done
clear
C)
urea and formaldehyde molecules
done
clear
D)
phenol and formaldehyde molecules
done
clear
View Answer play_arrow
question_answer 113) Chromatographic analysis is done based on the property of
A)
diffusion
done
clear
B)
absorption
done
clear
C)
adsorption
done
clear
D)
condensation
done
clear
View Answer play_arrow
question_answer 114) Avogadro number\[(6.023\times {{10}^{23}})\]of carbon atoms are present in
A)
12 g of\[^{12}C{{O}_{2}}\]
done
clear
B)
22.4 L\[^{12}C{{O}_{2}}\]at room temperature
done
clear
C)
44 g of \[^{12}C{{O}_{2}}\]
done
clear
D)
12 moles of \[^{12}C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 115) Glucose can be converted into ethyl alcohol using
A)
invertase
done
clear
B)
zymase
done
clear
C)
maltase
done
clear
D)
diastase
done
clear
View Answer play_arrow
question_answer 116) When\[FeC{{l}_{3}}\]is added to phenol
A)
no reaction occurs
done
clear
B)
a coloured complex will be formed
done
clear
C)
\[F{{e}^{3+}}\]will be oxidised to higher state
done
clear
D)
o-chlorophenol will be formed
done
clear
View Answer play_arrow
question_answer 117) The correct order of decreasing Lewis acidity is
A)
\[B{{F}_{3}}>BC{{l}_{3}}>BB{{r}_{3}}>B{{I}_{3}}\]
done
clear
B)
\[B{{I}_{3}}>BC{{l}_{3}}>BB{{r}_{3}}>B{{F}_{3}}\]
done
clear
C)
\[B{{I}_{3}}>BB{{r}_{3}}>BC{{l}_{3}}>B{{F}_{3}}\]
done
clear
D)
\[BC{{l}_{3}}>B{{F}_{3}}>BB{{r}_{3}}>B{{I}_{3}}\]
done
clear
View Answer play_arrow
question_answer 118) The buffer present in blood plasma is
A)
borax, sodium hydroxide
done
clear
B)
carbonic acid, bicarbonate ion
done
clear
C)
acetic acid, sodium acetate
done
clear
D)
citric acid, potassium dihydrogen phosphate
done
clear
View Answer play_arrow
question_answer 119) Conduction in a p-type semiconductor is increased by
A)
increasing the band gap
done
clear
B)
decreasing the temperature
done
clear
C)
adding appropriate electron deficient impurities
done
clear
D)
adding appropriate electron rich impurities
done
clear
View Answer play_arrow
question_answer 120) The volume of\[0.1\text{ }M\text{ }Ca{{(OH)}_{2}}\]required to neutralise 10 mL of\[0.1\text{ }N\text{ }HCl\]is
A)
10 mL
done
clear
B)
20 mL
done
clear
C)
5 mL
done
clear
D)
15 mL
done
clear
View Answer play_arrow
question_answer 121) Boron is unable to form\[BF_{6}^{3-}\]because of
A)
high electronegativity of boron
done
clear
B)
high electronegativity of fluorine
done
clear
C)
lack of d-orbitals in boron
done
clear
D)
less difference in electronegativity between B and F
done
clear
View Answer play_arrow
question_answer 122) The rate of reactions exhibiting negative activation energy
A)
decreases with increasing temperature
done
clear
B)
increases with increasing temperature
done
clear
C)
does not depend on temperature
done
clear
D)
depends on the height of the potential barrier
done
clear
View Answer play_arrow
question_answer 123) Addition of a non-volatile solute in a volatile ideal solvent
A)
increases the vapour pressure of the solvent
done
clear
B)
decreases the vapour pressure of the solvent
done
clear
C)
decreases the boiling point of the solvent
done
clear
D)
increases the freezing point of the solvent
done
clear
View Answer play_arrow
question_answer 124) The dissolution of a gas in a liquid is governed by
A)
Raoults law
done
clear
B)
Henrys law
done
clear
C)
Daltons law of partial pressure
done
clear
D)
vant Hoff factor
done
clear
View Answer play_arrow
question_answer 125) Normal human blood sugar range is 65-105 mg/dL. Considering density of human blood is 1.06 kg/L, if a patients sugar level reads 720 ppm, his/her blood sugar at that time is
A)
normal
done
clear
B)
high
done
clear
C)
low
done
clear
D)
cannot say
done
clear
View Answer play_arrow
question_answer 126) pH of 0.0002 M formic acid\[[{{K}_{a}}=2\times {{10}^{-4}}]\] approximately is
A)
1.35
done
clear
B)
0.5
done
clear
C)
3.7
done
clear
D)
1.85
done
clear
View Answer play_arrow
question_answer 127) At room temperature, for the reaction \[N{{H}_{4}}SH(s)N{{H}_{3}}(g)+{{H}_{2}}S(g)\]
A)
\[{{K}_{p}}={{K}_{c}}\]
done
clear
B)
\[{{K}_{p}}>{{K}_{c}}\]
done
clear
C)
\[{{K}_{p}}<{{K}_{c}}\]
done
clear
D)
\[{{K}_{p}}\]and\[{{K}_{c}}\]do not relate
done
clear
View Answer play_arrow
question_answer 128) The molecule NO is
A)
paramagnetic
done
clear
B)
diamagnetic
done
clear
C)
ferromagnetic
done
clear
D)
an even electron molecule
done
clear
View Answer play_arrow
question_answer 129) The most stable oxidation state exhibited by thallium is
A)
0
done
clear
B)
+1
done
clear
C)
+2
done
clear
D)
+3
done
clear
View Answer play_arrow
question_answer 130) Which of the following elements has the highest value of electron affinity?
A)
\[O\]
done
clear
B)
S
done
clear
C)
Se
done
clear
D)
Te
done
clear
View Answer play_arrow
question_answer 131) The correct order of electron gain enthalpy \[({{\Delta }_{eg}}H)\]of the halogen atoms is
A)
\[F<Cl<Br<I\]
done
clear
B)
\[Cl<F<Br<I\]
done
clear
C)
\[KBr<Cl<F\]
done
clear
D)
\[Cl<Br<I<F\]
done
clear
View Answer play_arrow
question_answer 132) Which of the following statements is correct?
A)
The equivalent mass of\[KMn{{O}_{4}}\]in alkaline medium is molar mass divided by five.
done
clear
B)
The equivalent mass of\[KMn{{O}_{4}}\]in strongly alkaline medium is molar mass divided by three.
done
clear
C)
The equivalent mass of\[KMn{{O}_{4}}\]in neutral medium is molar mass divided by three.
done
clear
D)
The equivalent mass of\[KMn{{O}_{4}}\]in weakly acidic medium is molar mass divided by three.
done
clear
View Answer play_arrow
question_answer 133) Bohr model of hydrogen atom was unable to explain
A)
Rydbergs formula of atomic spectra
done
clear
B)
Heisenbergs uncertainty principle
done
clear
C)
Plancks law of energy quantisation
done
clear
D)
Rutherfords model of atomic structure
done
clear
View Answer play_arrow
question_answer 134) The correct order of bond energy is
A)
\[C{{l}_{2}}>B{{r}_{2}}>{{F}_{2}}>{{I}_{2}}\]
done
clear
B)
\[C{{l}_{2}}>{{F}_{2}}>B{{r}_{2}}>{{I}_{2}}\]
done
clear
C)
\[{{I}_{2}}>B{{r}_{2}}>C{{l}_{2}}>{{F}_{2}}\]
done
clear
D)
\[{{I}_{2}}>B{{r}_{2}}>{{F}_{2}}>C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 135) The correct order of increasing oxidizing power in the series is
A)
\[VO_{2}^{+}<C{{r}_{2}}O_{7}^{2-}<MnO_{4}^{-}\]
done
clear
B)
\[C{{r}_{2}}O_{7}^{2-}<VO_{2}^{+}<MnO_{4}^{-}\]
done
clear
C)
\[C{{r}_{2}}O_{7}^{2-}<MnO_{4}^{-}>VO_{2}^{+}\]
done
clear
D)
\[MnO_{4}^{-}<C{{r}_{2}}O_{7}^{2-}<VO_{2}^{+}\]
done
clear
View Answer play_arrow
question_answer 136) Paramagnetism is shown by
A)
\[{{N}_{2}}\]
done
clear
B)
\[{{O}_{2}}\]
done
clear
C)
\[{{F}_{2}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 137) The spin only magnetic moment of \[{{[Cr{{F}_{6}}]}^{4-}}\](at. no. for Cr is 24) is
A)
0
done
clear
B)
1.73 BM
done
clear
C)
2.83BM
done
clear
D)
4.9BM
done
clear
View Answer play_arrow
question_answer 138) The reduction of zinc oxide with coke occurs at temperature
A)
greater than that for\[CuO\]
done
clear
B)
less than that for \[CuO\]
done
clear
C)
less than that for\[A{{g}_{2}}O\]
done
clear
D)
equal to that for \[CuO\]
done
clear
View Answer play_arrow
question_answer 139) The effective atomic number for \[{{[Rh{{({{H}_{2}}O)}_{6}}]}^{3+}}\] (at. no. for Rh is 45) is
A)
42
done
clear
B)
45
done
clear
C)
48
done
clear
D)
54
done
clear
View Answer play_arrow
question_answer 140) The crystal structure of solid Mn (II) oxide is
A)
\[NaCl\]structure
done
clear
B)
\[F{{e}_{2}}{{O}_{3}}\]structure
done
clear
C)
\[Ca{{F}_{2}}\]structure
done
clear
D)
\[N{{a}_{2}}O\]structure
done
clear
View Answer play_arrow
question_answer 141) The nitration (using nitration mixture) of aniline gives
A)
p-nitroaniline
done
clear
B)
o-nitroaniline
done
clear
C)
m-nitroaniline
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 142) The order of basic strength for methyl substituted amine in aqueous solution is
A)
\[N{{(C{{H}_{3}})}_{3}}>N{{(C{{H}_{3}})}_{2}}H>N(C{{H}_{3}}){{H}_{2}}\] \[>N{{H}_{3}}\]
done
clear
B)
\[N(C{{H}_{3}}){{H}_{2}}>N{{(C{{H}_{3}})}_{2}}H>N{{(C{{H}_{3}})}_{3}}\] \[>N{{H}_{3}}\]
done
clear
C)
\[N{{H}_{3}}_{2}>N(C{{H}_{3}}){{H}_{2}}>N{{(C{{H}_{3}})}_{2}}H\] \[>N{{(C{{H}_{3}})}_{3}}\]
done
clear
D)
\[N{{(C{{H}_{3}})}_{2}}H>N(C{{H}_{3}}){{H}_{2}}>N{{(C{{H}_{3}})}_{3}}\]\[>N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 143) The strongest acid among the choices is
A)
dichloroacetic acid
done
clear
B)
dimethylacetic acid
done
clear
C)
trifluoroacetic acid
done
clear
D)
triiodoacetic acid
done
clear
View Answer play_arrow
question_answer 144) Glucose and fructose can be distinguished by
A)
Lucas test
done
clear
B)
Ninhydrin test
done
clear
C)
Benedict reagent test
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 145) Which is the most stable compound among the following?
A)
done
clear
B)
done
clear
C)
done
clear
D)
All the compounds have same stability
done
clear
View Answer play_arrow
question_answer 146) Among the following, which is the least stable conformation of cyclohexane?
A)
Boat conformation
done
clear
B)
Half chair conformation
done
clear
C)
Twist boat conformation
done
clear
D)
Chair conformation
done
clear
View Answer play_arrow
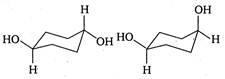
question_answer 147)
The correct relation between the following pair of compounds is
A)
constitutional isomers
done
clear
B)
enantiomers
done
clear
C)
diastereomers
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 148) Among the choices of alkyl bromide, the least reactive bromide in a\[{{S}_{N}}2\]reaction is
A)
1-bromopentane
done
clear
B)
2-bromo-2-methylbutane
done
clear
C)
1-bromo-3-methylbutane
done
clear
D)
1-bromo-2-methylbutane
done
clear
View Answer play_arrow
question_answer 149) The correct order of leaving group ability in a nucleophilic substitution reaction is
A)
\[B{{r}^{-}}>C{{l}^{-}}>C{{H}_{3}}CO_{2}^{-}>H{{O}^{-}}>{{H}^{-}}\]
done
clear
B)
\[{{H}^{-}}>O{{H}^{-}}>C{{H}_{3}}C{{O}_{2}}>C{{l}^{-}}>B{{r}^{-}}\]
done
clear
C)
\[B{{r}^{-}}>C{{H}_{3}}C{{O}_{2}}>CF>O{{H}^{-}}>{{H}^{-}}\]
done
clear
D)
\[C{{H}_{3}}C{{O}_{2}}>B{{r}^{-}}>C{{l}^{-}}>O{{H}^{-}}>{{H}^{-}}\]
done
clear
View Answer play_arrow
question_answer 150) 2-bromobutane reacts with\[O{{H}^{-}}\]in\[{{H}_{2}}O\]to give 2-butanol. The reaction involves
A)
retention in configuration
done
clear
B)
inversion in configuration
done
clear
C)
racemization
done
clear
D)
mutarotation
done
clear
View Answer play_arrow
question_answer 151) The plane of cell wall formation in a dividing cell is determined by
A)
Golgi apparatus
done
clear
B)
Microfilaments
done
clear
C)
microtubules
done
clear
D)
endoplasmic reticulum
done
clear
View Answer play_arrow
question_answer 152) From the following, select the statement that is true.
A)
All cells have a cell wall
done
clear
B)
Animals cells contain microtubules but plant cells do not contain microtubules
done
clear
C)
The Golgi apparatus is found only in animal cells
done
clear
D)
Chloroplasts are found in plant cells but not in prokaryotic or animal cells
done
clear
View Answer play_arrow
question_answer 153) The volume of air which remains in the conducting airways and is not available for gas exchange is called
A)
vital capacity
done
clear
B)
functional residual capacity
done
clear
C)
forced expiratory volume
done
clear
D)
anatomic dead space
done
clear
View Answer play_arrow
question_answer 154) The Darwinian fitness of an organism is a measure of
A)
its ability, relative to others in the population to pass its genes to the next generation
done
clear
B)
the number of offspring it produces
done
clear
C)
its lifespan
done
clear
D)
its physical vigour
done
clear
View Answer play_arrow
question_answer 155) A potential danger to a population that has been greatly reduced in number is the
A)
Hardy-Weinberg disequilibrium
done
clear
B)
tendency towards assortative mating
done
clear
C)
reduced gene flow
done
clear
D)
loss of genetic variability
done
clear
View Answer play_arrow
question_answer 156) An isolated population of humans with approximately equal numbers of blue-eyed and brown-eyed individuals was decimated by an earthquake. Only a few brown-eyed people remained to form the next generation. This kind of change in the gene pool is called a
A)
Hardy-Weinberg equilibrium
done
clear
B)
blocked gene flow
done
clear
C)
bottleneck effect
done
clear
D)
founder effect
done
clear
View Answer play_arrow
question_answer 157) The syndrome in humans in which individuals somatic cells contain the three sex chromosomes XXY is called
A)
Klinefelters syndrome
done
clear
B)
Turners syndrome
done
clear
C)
Downs syndrome
done
clear
D)
Superfemale
done
clear
View Answer play_arrow
question_answer 158) How does vaccination work?
A)
The immune system produces antibodies, which stay in the blood
done
clear
B)
Memory lymphocytes are produced. They remain in the body to fight off any future infection with the live pathogen
done
clear
C)
The dead pathogen stays in the body and constantly stimulates the immune system
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 159) The number of autosomes in a normal human cell is
A)
44
done
clear
B)
45
done
clear
C)
46
done
clear
D)
48
done
clear
View Answer play_arrow
question_answer 160) Downs syndrome is associated with trisomy of chromosome number
A)
20
done
clear
B)
21
done
clear
C)
22
done
clear
D)
23
done
clear
View Answer play_arrow
question_answer 161) Which of the following is the site of translation of the mRNA?
A)
Nucleus
done
clear
B)
Nucleolus
done
clear
C)
Golgi body
done
clear
D)
Ribosomes
done
clear
View Answer play_arrow
question_answer 162) Okazaki fragments are formed during
A)
transcription
done
clear
B)
translation
done
clear
C)
reverse transcription
done
clear
D)
DNA replication
done
clear
View Answer play_arrow
question_answer 163) Human proteins can be produced in the milk or semen of farm animals. True or false.
A)
True
done
clear
B)
False, proteins cannot be produced in milk.
done
clear
C)
False, proteins cannot be produced in semen
done
clear
D)
False, animals are not used for protein production
done
clear
View Answer play_arrow
question_answer 164) In a genetic engineering experiment, restriction enzymes can be used for
A)
bacterial DNA only
done
clear
B)
viral DNA only
done
clear
C)
any DNA fragment
done
clear
D)
eukaryotic DNA only
done
clear
View Answer play_arrow
question_answer 165) Which of the following is used to select genes of interest from a genomic library?
A)
Restriction enzymes
done
clear
B)
Cloning vectors
done
clear
C)
Gene targets
done
clear
D)
DNA probes
done
clear
View Answer play_arrow
question_answer 166) Sporopollenin, an organic material is present in
A)
stigma
done
clear
B)
style
done
clear
C)
exine
done
clear
D)
intine
done
clear
View Answer play_arrow
question_answer 167) In general, pollen tube enters the ovule through
A)
micropyle
done
clear
B)
chalaza
done
clear
C)
hilum
done
clear
D)
funicle
done
clear
View Answer play_arrow
question_answer 168) Transfer of pollen grain from anther to stigma of another flower of the same plant is called as
A)
geitonogamy
done
clear
B)
xenogamy
done
clear
C)
cleistogamy
done
clear
D)
chasmogamy
done
clear
View Answer play_arrow
question_answer 169) The endosperm cells in angiosperms are
A)
haploid
done
clear
B)
diploid
done
clear
C)
triploid
done
clear
D)
tetraploid
done
clear
View Answer play_arrow
question_answer 170) The fleshy edible part of an apple is
A)
thalamus
done
clear
B)
nucellus
done
clear
C)
ovary
done
clear
D)
endosperm
done
clear
View Answer play_arrow
question_answer 171) The portion of embryonal axis above cotyledon is called as
A)
epicotyl
done
clear
B)
hypocotyl
done
clear
C)
coleoptile
done
clear
D)
radicle
done
clear
View Answer play_arrow
question_answer 172) Phenotypic and genotypic ratio is similar in case of
A)
complete dominance
done
clear
B)
incomplete dominance
done
clear
C)
over dominance
done
clear
D)
epistasis
done
clear
View Answer play_arrow
question_answer 173) Which of the following is the number of alleles for blood group in an individual?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 174) Cholesterol belongs to which of the following groups?
A)
Steroids
done
clear
B)
Neutral fats
done
clear
C)
Waxes
done
clear
D)
Phospholipids
done
clear
View Answer play_arrow
question_answer 175) Which of the following occurs at the ribosomes?
A)
Most of a cells DNA molecules are stored there
done
clear
B)
Proteins are produced there
done
clear
C)
mRNAs are produced there
done
clear
D)
DNA replication takes place there
done
clear
View Answer play_arrow
question_answer 176) Which of the following is the ultimate source of energy in an ecosystem?
A)
Sunlight
done
clear
B)
Producers
done
clear
C)
Consumers
done
clear
D)
Decomposers
done
clear
View Answer play_arrow
question_answer 177) The interaction where one species is benefitted and the other is neither benefitted nor harmed is called as
A)
amensalism
done
clear
B)
commensalism
done
clear
C)
mutualism
done
clear
D)
predation
done
clear
View Answer play_arrow
question_answer 178) The detritus food chain begins with
A)
primary producers
done
clear
B)
primary consumers
done
clear
C)
secondary consumers
done
clear
D)
dead organic matter
done
clear
View Answer play_arrow
question_answer 179) The problem of electrical discontinuity caused in the normal heart by the connective tissue separating the atria from the ventricles is solved by
A)
coordinating electrical activity in the atria with electrical activity in the ventricles by connecting them via the boundle of His
done
clear
B)
having the AV node function as a secondary pacemaker
done
clear
C)
having an ectopic pacemaker
done
clear
D)
coordinating electrical activity in the atria with electrical activity in the ventricles by connecting them via the vagus nerve
done
clear
View Answer play_arrow
question_answer 180) The protein whose removal enables myosin to bind actin in smooth muscle is
A)
tropomyosin
done
clear
B)
caldesmon
done
clear
C)
myosin light chain kinase
done
clear
D)
calmodulin
done
clear
View Answer play_arrow
question_answer 181) An investigator places an isolated neuron in a calcium-free medium, gives the neuron a suprathreshold stimulus and then performs an assay to test whether neurotransmitter is released into the medium. Which of the following outcomes would you predict?
A)
No neurotransmitter is detected since influx of calcium into the synaptic knob is required for neurotransmitter release
done
clear
B)
No neurotransmitter is detected since influx of calcium is required in order for the neuron to conduct an action potential
done
clear
C)
Neurotransmitter is detected since calcium is not required for action potential conduction and the initial stimulus was suprathreshold
done
clear
D)
We cannot predict the outcome without knowing whether the neuron was myelinated
done
clear
View Answer play_arrow
question_answer 182) What product of the immune system attaches to bacteria, making them easier to be eaten by white blood cells?
A)
Antigen
done
clear
B)
Haemoglobin
done
clear
C)
Antibody
done
clear
D)
MHC I molecule
done
clear
View Answer play_arrow
question_answer 183) A patient with symptoms of allergies would have an elevation of which of the following?
A)
\[IgE\]
done
clear
B)
White blood count
done
clear
C)
\[IgM\]
done
clear
D)
\[IgD\]
done
clear
View Answer play_arrow
question_answer 184) In anaphylactic shock, a substance is released which causes dilation of the blood vessels and capillary leaking. What is this substance called?
A)
Adrenaline
done
clear
B)
Benadryl
done
clear
C)
Albumin
done
clear
D)
Histamine
done
clear
View Answer play_arrow
question_answer 185) Pear fruits are gritty due to the presence of
A)
tracheids
done
clear
B)
vessels
done
clear
C)
fibres
done
clear
D)
sclereids
done
clear
View Answer play_arrow
question_answer 186) Which of the following growth hormones is associated with stomatal movements?
A)
Auxin
done
clear
B)
Gibberellin
done
clear
C)
ABA
done
clear
D)
Cytokinin
done
clear
View Answer play_arrow
question_answer 187) Denitrification is carried out by
A)
Pseudomonas
done
clear
B)
Nitrobacter
done
clear
C)
Nitrosomonas
done
clear
D)
Nitrococcus
done
clear
View Answer play_arrow
question_answer 188) Non-cyclic photophosphorylation results in the production of
A)
ADP
done
clear
B)
ATP
done
clear
C)
NADPH
done
clear
D)
ATP and NADPH
done
clear
View Answer play_arrow
question_answer 189) The site of glycolysis is
A)
cytoplasm
done
clear
B)
chloroplast
done
clear
C)
mitochondrial matrix
done
clear
D)
mitochondrial inner membrane
done
clear
View Answer play_arrow
question_answer 190) The two organisms which breathe only through their moist skin are
A)
fish and frog
done
clear
B)
frog and earthworm
done
clear
C)
leech and earthworm
done
clear
D)
fish and earthworm
done
clear
View Answer play_arrow
question_answer 191) The alpha helices and beta sheets are the example of which level of protein organization?
A)
Primary structure
done
clear
B)
Secondary structure
done
clear
C)
Tertiary structure
done
clear
D)
Quaternary structure
done
clear
View Answer play_arrow
question_answer 192) Cervical cancer can be caused by
A)
Chlamydia sp.
done
clear
B)
Human papilloma virus
done
clear
C)
Herpes simplex virus
done
clear
D)
Neisseria gonorrhoeae
done
clear
View Answer play_arrow
question_answer 193) In human females, the ovarian cycle begins when the
A)
levels of oestrogen reach their maximum
done
clear
B)
hypothalamus stimulates the anterior pituitary to increase its output of FSH and LH
done
clear
C)
level of progesterone drops precipitously
done
clear
D)
hypothalamus increases its release-of FSH and LH
done
clear
View Answer play_arrow
question_answer 194) A vasectomy
A)
prevents the production of sperm in the testes
done
clear
B)
prevents the production of semen
done
clear
C)
prevents the movement of sperm into the urethra
done
clear
D)
prevents a man from having an erection
done
clear
View Answer play_arrow
question_answer 195) Sperm of animal species A cannot fertilize ovum of species B because
A)
fertilizin of A and antifertilizin of B are not compatible
done
clear
B)
antifertilizin of A and fertilizin of B are not compatible
done
clear
C)
fertilizin of A and B are not compatible
done
clear
D)
antifertilizin of A and B are not compatible
done
clear
View Answer play_arrow
question_answer 196) The unit of evolution is now known to be the
A)
individual
done
clear
B)
family
done
clear
C)
populaton
done
clear
D)
species
done
clear
View Answer play_arrow
question_answer 197) Trichoderma is an example of which of the following?
A)
Phycomycetes
done
clear
B)
Zygomycetes
done
clear
C)
Deuteromycetes
done
clear
D)
Basidiomycetes
done
clear
View Answer play_arrow
question_answer 198) Lichen is an association between
A)
fungi and bryophyte
done
clear
B)
fungi and algae
done
clear
C)
algae and pteridophyte
done
clear
D)
algae and bacteria
done
clear
View Answer play_arrow
question_answer 199) The genetic material of a viroid is
A)
DNA
done
clear
B)
RNA
done
clear
C)
protein
done
clear
D)
carbohydrate
done
clear
View Answer play_arrow
question_answer 200) Mannitol is a stored food material found in members of which of the following classes?
A)
Chlorophyceae
done
clear
B)
Xanthophyceae
done
clear
C)
Rhodophyceae
done
clear
D)
Phaeophyceae
done
clear
View Answer play_arrow
question_answer 201) The first stable product of\[{{C}_{4}}-\]pathway is
A)
OAA
done
clear
B)
PGA
done
clear
C)
PGAL
done
clear
D)
DHAP
done
clear
View Answer play_arrow
question_answer 202) Energy equivalent of a NADH is which of the following numbers of ATP molecules?
A)
2
done
clear
B)
3
done
clear
C)
38
done
clear
D)
36
done
clear
View Answer play_arrow
question_answer 203) Internodal elongation is associated with
A)
auxin
done
clear
B)
cytokinin
done
clear
C)
gibberellin
done
clear
D)
ABA
done
clear
View Answer play_arrow
question_answer 204) Which of the following is not a characteristic features of arthropods?
A)
Jointed appendages
done
clear
B)
Unsegmented body
done
clear
C)
Moulting
done
clear
D)
Articulated exoskeleton
done
clear
View Answer play_arrow
question_answer 205) Obliquely placed ovary and swollen placenta is associated with which of the following families?
A)
Asteraceae
done
clear
B)
Solanaceae
done
clear
C)
Brassicaceae
done
clear
D)
Malvaceae
done
clear
View Answer play_arrow
question_answer 206) On the basis of position of the ovary, mustard plants are
A)
hypogynous
done
clear
B)
perigynous
done
clear
C)
epigynous
done
clear
D)
zygomorphic
done
clear
View Answer play_arrow
question_answer 207) The flower of Calotropis has which of the following aestivations?
A)
Twisted
done
clear
B)
Imbricate
done
clear
C)
Valvate
done
clear
D)
Vexillary
done
clear
View Answer play_arrow
question_answer 208) The population limited to a particular geographic area is called as
A)
pandemic
done
clear
B)
endemic
done
clear
C)
alien
done
clear
D)
natural
done
clear
View Answer play_arrow
question_answer 209) Which of the following has the largest population in a food chain?
A)
Producers
done
clear
B)
Primary consumers
done
clear
C)
Secondary consumers
done
clear
D)
Decomposers
done
clear
View Answer play_arrow
question_answer 210) The second trophic level of longer food chains in a lake is
A)
phytoplankton
done
clear
B)
zooplankton
done
clear
C)
benthos
done
clear
D)
fishes
done
clear
View Answer play_arrow
question_answer 211) The vertical distribution of different species occupying different levels is called as
A)
stratification
done
clear
B)
fragmentation
done
clear
C)
mobilization
done
clear
D)
mineralization
done
clear
View Answer play_arrow
question_answer 212) Widal test is specific for the diagnosis of which of the following diseases?
A)
Typhoid
done
clear
B)
Malaria
done
clear
C)
Pneumonia
done
clear
D)
Common cold
done
clear
View Answer play_arrow
question_answer 213) Antibodies resemble which of the following shape?
A)
X
done
clear
B)
Y
done
clear
C)
Z
done
clear
D)
O
done
clear
View Answer play_arrow
question_answer 214) AIDS is caused by a
A)
retrovirus
done
clear
B)
DNA virus
done
clear
C)
viroid
done
clear
D)
protein
done
clear
View Answer play_arrow
question_answer 215) Which of the following belongs to the class- Gastropoda?
A)
Clam
done
clear
B)
Cuttle fish
done
clear
C)
Snail
done
clear
D)
Mussel
done
clear
View Answer play_arrow
question_answer 216) The blood-brain barrier
A)
consists of both anatomical and physiological factors
done
clear
B)
regulates to some extent the passage of substances from the blood to the interstitial fluid of the brain
done
clear
C)
is anatomically related to the formation of tight junctions between adjacent capillary endothelial cells
done
clear
D)
All of the above are correct
done
clear
View Answer play_arrow
question_answer 217) The stages between larval moults in an insects are called
A)
pupae
done
clear
B)
instars
done
clear
C)
grubs
done
clear
D)
caterpillars
done
clear
View Answer play_arrow
question_answer 218) Which of the following animals is a reptile?
A)
Salamander
done
clear
B)
Toad
done
clear
C)
Newt
done
clear
D)
Turtle
done
clear
View Answer play_arrow
question_answer 219) The secretion of tears, milk, sweat and oil are functions of which of the following tissues?
A)
Epithelial
done
clear
B)
Nervous
done
clear
C)
Loose connective
done
clear
D)
Lymphoid
done
clear
View Answer play_arrow
question_answer 220) Collagen fibers are characteristic of which tissue?
A)
Muscle
done
clear
B)
Epithelial
done
clear
C)
Connective
done
clear
D)
Nervous
done
clear
View Answer play_arrow
question_answer 221) Cortisol is secreted by the adrenal cortex in response to stress. In addition to its function in a stress response, it functions in negative feedback by
A)
inhibiting the hypothalamus so that Corticotropin Releasing Hormone (CRH) secretion is reduced
done
clear
B)
inhibiting the anterior pituitars ability to respond to CRH by reducing the pituitarys sensitivity to CRH
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 222) Why asexual reproduction is sometimes disadvantageous?
A)
It allows animals that do not move around to produce offspring without finding mates
done
clear
B)
It allows an animal to produce many offspring quickly
done
clear
C)
It saves the time and energy of gamete production
done
clear
D)
It produces genetically uniform populations
done
clear
View Answer play_arrow
question_answer 223) Which of the following is responsible for nourishing the developing sperm?
A)
Sertoli cells
done
clear
B)
Leydig cells
done
clear
C)
Granulosa cells
done
clear
D)
Corpus luteum
done
clear
View Answer play_arrow
question_answer 224) What is the site of fertilization in mammals?
A)
Cervix
done
clear
B)
Uterus
done
clear
C)
Vagina
done
clear
D)
Fallopian tubes
done
clear
View Answer play_arrow
question_answer 225) Surfactant
A)
is a protein produced by type II alveolar cells
done
clear
B)
is excessive in many premature infants resulting in difficulties in breathing
done
clear
C)
Decreases the surface tension of the fluid lining the alveoli
done
clear
D)
is lacking in individuals suffering from acute respiratory distress syndrome
done
clear
View Answer play_arrow
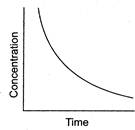 What is the expected order for such reactions?
What is the expected order for such reactions?