question_answer 1) The kinetic energy and potential energy of a re executing simple harmonic motion will be equal, when displacement (amplitude = a) is:
A)
\[a\sqrt{2}\]
done
clear
B)
\[\frac{a}{2}\]
done
clear
C)
\[\frac{a\sqrt{2}}{3}\]
done
clear
D)
\[\frac{a}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 2) When an aeroplane moves at a speed higher, than the velocity of sound in air, a loud bang is heard. This is because :
A)
it explodes
done
clear
B)
it produces shock wave which is received as the bang
done
clear
C)
its wings vibrate so violently that the bang is heard
done
clear
D)
the normal engine noise undergoes a Doppler shift to generate the bang
done
clear
View Answer play_arrow
question_answer 3) Two waves of the same frequency but of amplitudes in the ratio 1 : 3 are superimposed. The ratio of maximum to minimum intensity is:
A)
4 : 1
done
clear
B)
1 : 4
done
clear
C)
3 : 1
done
clear
D)
1 : 3
done
clear
View Answer play_arrow
question_answer 4) A vessel, whose bottom has round holes with diameter of 1 mm is filled with water. Assuming that surface tension acts only at holes, then the maximum height to which the water can be filled in vessel without leakage is: (Given, surface tension of water is \[75\times {{10}^{-3}}N/m\]and \[g=10\text{ }m/{{s}^{2}}\])
A)
3 cm
done
clear
B)
0.3 cm
done
clear
C)
3 mm
done
clear
D)
3 m
done
clear
View Answer play_arrow
question_answer 5) The electric field inside a spherical shell of uniform surface charge density is :
A)
zero
done
clear
B)
constant less than zero
done
clear
C)
directly proportional to distance from the centre
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 6) If the distance between the plates of a parallel plate condenser of capacity 10\[\mu F\] is doubled, then new capacity will be :
A)
5 \[\mu F\]
done
clear
B)
20\[\mu F\]
done
clear
C)
10\[\mu F\]
done
clear
D)
15\[\mu F\]
done
clear
View Answer play_arrow
question_answer 7) In the fission of uranium, 0.5 g mass is decayed, then how much energy will be obtained by it?
A)
\[1.25\text{ }kWh\]
done
clear
B)
\[1.25\times {{10}^{7}}kWh\]
done
clear
C)
\[0.25\text{ }kWh\]
done
clear
D)
\[1.25\times {{10}^{4}}kWh\]
done
clear
View Answer play_arrow
question_answer 8) Photocells are used for the :
A)
reproduction of films from the cinema films
done
clear
B)
reproduction of sound from the cinema films
done
clear
C)
automatic switching of street light
done
clear
D)
both (a) and (b)
done
clear
View Answer play_arrow
question_answer 9) Photoelectric effect can be explained by assuming that light:
A)
is a form of transverse wave
done
clear
B)
is a form of longitudinal wave
done
clear
C)
can be polarized
done
clear
D)
consists of quanta
done
clear
View Answer play_arrow
question_answer 10) The work function of a metal 2.8 eV. Its threshold wavelength will be :
A)
4400\[\overset{0}{\mathop{A}}\,\]
done
clear
B)
4433 \[\overset{0}{\mathop{A}}\,\]
done
clear
C)
5434 \[\overset{0}{\mathop{A}}\,\]
done
clear
D)
433 \[\overset{0}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 11) The stopping potential depends on :
A)
intensity of incident light
done
clear
B)
frequency of incident light
done
clear
C)
both (a) and (b)
done
clear
D)
neither nor
done
clear
View Answer play_arrow
question_answer 12) An oil drop of radius \[{{10}^{-6}}m\]carries charge equal to that of 3 electrons. If density of the oil is \[2\times {{10}^{3}}kg-{{m}^{3}},\] then electric field required to keep the drop stationary is:
A)
\[1.71\times {{10}^{5}}V{{m}^{-1}}\]
done
clear
B)
\[1.71\times {{10}^{3}}V{{m}^{-1}}\]
done
clear
C)
\[1.71\times {{10}^{6}}V{{m}^{-1}}\]
done
clear
D)
\[~1.71\text{ }V{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 13) What is the acceleration of an electric field of magnitude \[50\text{ }V-c{{m}^{-1}}\]? [Given that\[\frac{e}{m}\] \[=\text{ }1.76\text{ }x\text{ }{{10}^{11}}C-k{{g}^{-1}}\]]
A)
\[8.8\times {{10}^{16}}m/{{s}^{2}}\]
done
clear
B)
\[8.8\times {{10}^{14}}m/{{s}^{2}}\]
done
clear
C)
\[8.8\text{ }m/{{s}^{2}}\]
done
clear
D)
\[8.8\times {{10}^{20}}m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 14) Light waves can be polarised because they :
A)
have high frequency
done
clear
B)
have high wavelength
done
clear
C)
are transverse
done
clear
D)
can be reflected
done
clear
View Answer play_arrow
question_answer 15) In which one of the following substances the resistance decreases with increase of temperature?
A)
Carbon
done
clear
B)
Copper
done
clear
C)
Silver
done
clear
D)
Gold
done
clear
View Answer play_arrow
question_answer 16) A heater coil rated at (1000W-200V) is connected to 110 V line. What will be the power consumed?
A)
200 W
done
clear
B)
300 W
done
clear
C)
250 W
done
clear
D)
350 W
done
clear
View Answer play_arrow
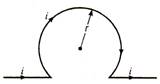
question_answer 17)
An infinitely long straight conductor is bent into the shape as shown in figure. It carries a current of i ampere and the radius of the circular loop is r metre, then the magnetic induction at the centre of the circular part is :
A)
zero
done
clear
B)
infinite
done
clear
C)
\[\frac{2{{\mu }_{0}}i(\pi +1)}{4\pi r}\]
done
clear
D)
\[\frac{2{{\mu }_{0}}i(\pi -1)}{4\pi r}\]
done
clear
View Answer play_arrow
question_answer 18) Field inside a solenoid is :
A)
directly proportional to its length
done
clear
B)
directly proportional to current
done
clear
C)
inversely proportional to number of turns
done
clear
D)
inversely proportional to current
done
clear
View Answer play_arrow
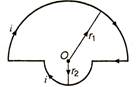
question_answer 19)
In the given figure there are two semicircles of radii r1 and r2 in which a current i is flowing. The magnetic induction at centre O will be :
A)
\[\frac{{{\mu }_{0}}i({{r}_{1}}+{{r}_{2}})}{4}\]
done
clear
B)
\[\frac{{{\mu }_{0}}i({{r}_{1}}-{{r}_{2}})}{4}\]
done
clear
C)
\[\frac{{{\mu }_{0}}i({{r}_{1}}+{{r}_{2}})}{4{{r}_{1}}{{r}_{2}}}\]
done
clear
D)
\[\frac{{{\mu }_{0}}i({{r}_{2}}-{{r}_{1}})}{4{{r}_{1}}{{r}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 20) When a magnet is heated :
A)
it loses its magnetism
done
clear
B)
it gains magnetism
done
clear
C)
neither loses nor gains magnetism
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 21) At Curie point, a ferromagnetic materic becomes :
A)
non-magnetic
done
clear
B)
diamagnetic
done
clear
C)
paramagnetic
done
clear
D)
strongly ferromagnetic
done
clear
View Answer play_arrow
question_answer 22) A capacitor is perfectly insulator for :
A)
direct current
done
clear
B)
alternating current
done
clear
C)
direct as well as alternating current
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 23) Alternating current is converted into direr current by :
A)
dynamo
done
clear
B)
motor
done
clear
C)
transformer
done
clear
D)
rectifier
done
clear
View Answer play_arrow
question_answer 24) The inductive reactance of an inductor in an AC circuit is :
A)
\[\frac{1}{\omega L}\]
done
clear
B)
\[\frac{\omega }{L}\]
done
clear
C)
\[\frac{L}{\omega }\]
done
clear
D)
\[\omega L\]
done
clear
View Answer play_arrow
question_answer 25) The average power dissipated in a pure inductor of inductance L is :
A)
\[\frac{L{{I}^{2}}}{2}\]
done
clear
B)
\[L{{I}^{2}}\]
done
clear
C)
\[\frac{L{{I}^{2}}}{4}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 26) The electromagnetic waves travel with a velocity :
A)
equal to velocity of sound
done
clear
B)
equal to velocity of light
done
clear
C)
less than velocity of light
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 27) Two sources of light (\[{{S}_{1}}\] and \[{{S}_{2}}\]) of wave ie r: jib 2500\[\overset{0}{\mathop{A}}\,\] and 3500\[\overset{0}{\mathop{A}}\,\] respectively are used in Youngs double slit experiment simultaneously Find out at which order of two wave trail pattern the fringes coincide.
A)
Fifth order fringe due to \[{{S}_{1}}\] will coincide with seventh order fringe due to \[{{S}_{2}}\]
done
clear
B)
Fifth order fringe due to \[{{S}_{2}}\]will coincide with seventh order fringe due to
done
clear
C)
Seventh order fringe due to \[{{S}_{1}}\] will coincide with sixth order fringe due to \[{{S}_{2}}\]
done
clear
D)
Seventh order fringe due to \[{{S}_{2}}\] will coincide with sixth order fringe due to \[{{S}_{1}}\]
done
clear
View Answer play_arrow
question_answer 28) Light travels faster in air, than in glass according to :
A)
wave theory of light
done
clear
B)
corpuscular theory of light
done
clear
C)
both (a) and (b)
done
clear
D)
neither nor
done
clear
View Answer play_arrow
question_answer 29)
Four independent waves are expressed as : (i) \[{{y}_{1}}={{a}_{1}}\sin (\omega t)\] (ii) \[{{y}_{2}}={{a}_{2}}\sin (2\omega t)\] (iii) \[{{y}_{3}}={{a}_{3}}\cos (\omega t)\] (iv)\[{{y}_{4}}={{a}_{4}}\sin (\omega t+\frac{\pi }{3})\]
The interference is possible between:
A)
(i) and (iii)
done
clear
B)
(i) and (iv)
done
clear
C)
(iii) and (iv)
done
clear
D)
not possible at all
done
clear
View Answer play_arrow
question_answer 30) When light waves suffer reflection at the interface between air and glass, the change of phase of the reflected wave is equal to :
A)
zero
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\pi \]
done
clear
D)
2\[\pi \]
done
clear
View Answer play_arrow
question_answer 31) The power of lens, a short sighted person uses is -2D. Find the maximum distance of an object, which he can see without spectacle.
A)
25 cm
done
clear
B)
50 cm
done
clear
C)
100 cm
done
clear
D)
10 cm
done
clear
View Answer play_arrow
question_answer 32) Two thin long parallel wires separated by a distance b are carrying a current i amperes each. The magnitude of the force per unit length exerted by one wire on the other is :
A)
\[\frac{{{\mu }_{0}}{{i}^{2}}}{{{b}^{2}}}\]
done
clear
B)
\[\frac{{{\mu }_{0}}{{i}^{2}}}{2\pi b}\]
done
clear
C)
\[\frac{{{\mu }_{0}}i}{2\pi b}\]
done
clear
D)
\[\frac{{{\mu }_{0}}{{i}^{2}}}{2\pi {{b}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 33) The quantity of electricity that deposits 1 g equivalent of substance is called :
A)
farad
done
clear
B)
faraday
done
clear
C)
coulomb
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 34) Kirchhoffs first law, i.e., \[\sum i=0\] at a junction deals with conservation of:
A)
charges
done
clear
B)
energy
done
clear
C)
momentum
done
clear
D)
angular momentum
done
clear
View Answer play_arrow
question_answer 35) To observe diffraction, the size of an obstacle :
A)
should be of the same order as wavelength
done
clear
B)
should be much larger than the wavelength
done
clear
C)
have no relation with wavelength
done
clear
D)
should be exactly
done
clear
View Answer play_arrow
question_answer 36) In a Thomson setup, \[E=30V-c{{m}^{-1}}\]and B = 6 G. Then, speed of the electron that goes undeflected in the common region of the two fields will be :
A)
\[15\times {{10}^{6}}m/s\]
done
clear
B)
\[25\times {{10}^{6}}m/s\]
done
clear
C)
\[5\times {{10}^{6}}\text{ }m/s\]
done
clear
D)
\[1\times {{10}^{6}}\text{ }m/s\]
done
clear
View Answer play_arrow
question_answer 37) In a gas equation, for one mole of real gas\[\left( p+\frac{a}{{{V}^{2}}} \right)(V-b)=RT\], dimensions of constants a and b are :
A)
\[[{{L}^{2}}],[M{{L}^{4}}{{T}^{-2}}]\]
done
clear
B)
\[[L],[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
C)
\[[M{{L}^{5}}{{T}^{-2}}],[{{L}^{3}}]\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
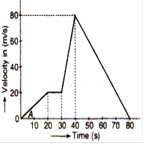
question_answer 38)
The velocity time graph of moving object is given in the figure. Find the maximum acceleration of the body and distance travelled by the body in the interval of time in which this acceleration exists.
A)
\[6\text{ }m/{{s}^{2}},500\text{ }m\]
done
clear
B)
\[3\text{ }m/{{s}^{2}},200m\]
done
clear
C)
\[-6\text{ }m/{{s}^{2}},500m\]
done
clear
D)
\[-3\text{ }m/{{s}^{2}},500m\]
done
clear
View Answer play_arrow
question_answer 39) A cyclist riding at a speed of 9.8 m/s takes a turn around a circular road of radius 19.6 m. What is his inclination to the vertical?
A)
\[30{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[10.5{}^\circ \]
done
clear
D)
\[26.5{}^\circ \]
done
clear
View Answer play_arrow
question_answer 40) A man weighs 60 kg at earth surface. At what height above the earths surface weight becomes 30 kg? (Given, radius of earth is 6400 km.)
A)
2624 km
done
clear
B)
3000 km
done
clear
C)
2020 km
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 41) As we go from the equator to the poles the value of acceleration due to gravity :
A)
remains the same
done
clear
B)
increases
done
clear
C)
decreases
done
clear
D)
decreases upto a latitude of 45° and then increases
done
clear
View Answer play_arrow
question_answer 42) When torque acting upon a system is zero which of the following will be constant?
A)
Force
done
clear
B)
Linear momentum
done
clear
C)
Linear impulse
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 43) If a gymnast sitting on a rotating stool with his arms outstretched, suddenly lowers his hands :
A)
the angular velocity decreases
done
clear
B)
his moment of inertia decreases
done
clear
C)
the angular velocity stays constant
done
clear
D)
the angular momentum increases
done
clear
View Answer play_arrow
question_answer 44) A force of 40 N is acting between two charges in air. If the space between them is filled with glass with \[{{\varepsilon }_{r}}=8\], what will be the force?
A)
15 N
done
clear
B)
320 N
done
clear
C)
5 N
done
clear
D)
32 N
done
clear
View Answer play_arrow
question_answer 45) Two glass plates having a little water in between cannot be easily separated because of:
A)
viscosity
done
clear
B)
atmospheric pressure
done
clear
C)
surface tension
done
clear
D)
friction
done
clear
View Answer play_arrow
question_answer 46) Good absorbers of heat are :
A)
poor emitters
done
clear
B)
non-emitter
done
clear
C)
good emitters
done
clear
D)
highly polished
done
clear
View Answer play_arrow
question_answer 47) The work done in increasing the temperature of 0.2 mole of nitrogen at constant pressure from \[37{}^\circ C\] to \[337{}^\circ C\] is : \[({{C}_{p}}=7cal/mol-{}^\circ C)\]
A)
1764 J
done
clear
B)
1600 J
done
clear
C)
160 J
done
clear
D)
176 J
done
clear
View Answer play_arrow
question_answer 48) A particle has displacement y given by y = 3 \[\sin (r\pi t+\pi )\], where y is in metre and t in second. What are frequency and period of motion?
A)
0.4 Hz, 2.5 s
done
clear
B)
2.5 Hz, 0.4 s
done
clear
C)
2.5 Hz, 2.5 s
done
clear
D)
0.4 Hz, 0.4 s
done
clear
View Answer play_arrow
question_answer 49) A spring having a spring constant k is loaded with a mass m. The spring is cut into two parts and one of them is loaded again with same mass. The new constant is :
A)
\[\frac{k}{2}\]
done
clear
B)
k
done
clear
C)
2k
done
clear
D)
k2
done
clear
View Answer play_arrow
question_answer 50) In a sonometer wire, the produced waves an
A)
longitudinal
done
clear
B)
transverse, stationary and unpolarizec
done
clear
C)
transverse, stationary and polarized
done
clear
D)
transverse, progressive and polarized
done
clear
View Answer play_arrow
question_answer 51) Beats are the result of:
A)
diffraction
done
clear
B)
destructive interference
done
clear
C)
constructive and destructive interferer.:*
done
clear
D)
superposition of two waves of nearly frequencies
done
clear
View Answer play_arrow
question_answer 52) An electron is sent in electric field of intensity \[9.1\times {{10}^{6}}N/C.\] The acceleration produce:
A)
\[1.6\text{ }m/{{s}^{2}}\]
done
clear
B)
\[1.6\times {{10}^{18}}m/{{s}^{2}}\]
done
clear
C)
\[3.2\times {{10}^{18}}m/{{s}^{2}}\]
done
clear
D)
\[0.8\times {{10}^{18}}m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 53) The empty space between the plates capacitor is filled by a liquid of constant K. The capacitance of capacitor:
A)
increases by a factor \[{{K}^{2}}\]
done
clear
B)
increases by a factor K
done
clear
C)
decreases by a-factor \[{{K}^{2}}\]
done
clear
D)
decreases by a factor K
done
clear
View Answer play_arrow
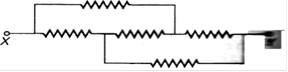
question_answer 54)
What is the resistance between the X and Y of the circuit in the figure ? that the resistance of each resistor 25\[\Omega \] .
A)
100 \[\Omega \]
done
clear
B)
50\[\Omega \]
done
clear
C)
25 \[\Omega \]
done
clear
D)
75\[\Omega \]
done
clear
View Answer play_arrow
question_answer 55) At what temperature is rms : molecules double of that at NTP
A)
\[819{}^\circ C\]
done
clear
B)
\[719{}^\circ C\]
done
clear
C)
\[909{}^\circ C\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 56) The dimensions of the quantities in one of the following pairs are the same. Identify the pairs.
A)
Torque and work
done
clear
B)
Angular momentum and work
done
clear
C)
Energy and Youngs modulus
done
clear
D)
Light year and time
done
clear
View Answer play_arrow
question_answer 57) Position of a particle moving along x-axis is given by \[x=3t-4{{t}^{2}}+{{t}^{3}},\] where x is in metre and t in seconds. Find the average velocity of the particle in the time interval from t = 2 s to 4 s.
A)
7 m/s
done
clear
B)
9 m/s
done
clear
C)
13 m/s
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 58) Velocity is :
A)
scalar
done
clear
B)
vector
done
clear
C)
neither scalar nor vector
done
clear
D)
both scalar and vector
done
clear
View Answer play_arrow
question_answer 59) You are on a frictionless horizontal plane. How can you get off, if no horizontal force is exerted by pushing against the surface?
A)
By jumping
done
clear
B)
By spitting or sneezing
done
clear
C)
By rolling your body on the surface
done
clear
D)
By running on the plane
done
clear
View Answer play_arrow
question_answer 60) At the top of the trajectory of a projectile, the directions of its velocity and acceleration are :
A)
perpendicular to each other
done
clear
B)
parallel to each other
done
clear
C)
inclined to each other at an angle of 45°
done
clear
D)
antiparallel to each other
done
clear
View Answer play_arrow
question_answer 61) A car moving at 30 m/s slows uniformly to a speed of 10 m/s in a time of 5 s. Determine the distance moved in the third second.
A)
10 m
done
clear
B)
20 m
done
clear
C)
40 m
done
clear
D)
5m
done
clear
View Answer play_arrow
question_answer 62) The Poiseuille is the unit of:
A)
pressure
done
clear
B)
friction
done
clear
C)
surface tension
done
clear
D)
viscosity
done
clear
View Answer play_arrow
question_answer 63) If the radius of the earth contracts to half of its present day value without change in mass, what will be the length of the day?
A)
24 h
done
clear
B)
48 h
done
clear
C)
6 h
done
clear
D)
12 h
done
clear
View Answer play_arrow
question_answer 64) The gravitational force with which the earth attracts the moon :
A)
is less than the force with which the moon attracts the earth
done
clear
B)
is equal to the force with which the moon attracts the earth
done
clear
C)
is twice than the force with which the moon attracts the earth
done
clear
D)
varies with the phases of moon
done
clear
View Answer play_arrow
question_answer 65) A 2.5 kg mass moving at a speed of 15 m/s collides with 5 kg object initially at rest. They stick together. Find the velocity of the combination after the collision.
A)
15 m/s
done
clear
B)
5 m/s
done
clear
C)
20 m/s
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 66) A geostationary satellite :
A)
revolve about the polar axis
done
clear
B)
has a time period less than that of the near earth satellite
done
clear
C)
moves faster than a near earth satellite
done
clear
D)
is stationary in the space
done
clear
View Answer play_arrow
question_answer 67) What will be the angle of contact of a liquid relative to a solid, if forces of adhesion and cohesion are equal?
A)
\[0{}^\circ \]
done
clear
B)
\[30{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 68) The electric charge in uniform motion produces:
A)
an electric field only
done
clear
B)
a magnetic field only
done
clear
C)
both electric and magnetic fields
done
clear
D)
neither electric nor magnetic field
done
clear
View Answer play_arrow
question_answer 69) A copper disc with a central hole is heated. The diameter of the hole :
A)
increases
done
clear
B)
decreases
done
clear
C)
first decreases then increases
done
clear
D)
remains unchanged
done
clear
View Answer play_arrow
question_answer 70) For motion of a simple pendulum to be simple harmonic :
A)
amplitude of motion will be very small
done
clear
B)
amplitude of motion will be very large
done
clear
C)
independent of amplitude of motion
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 71)
The given truth table is of:
A)
OR gate
done
clear
B)
AND gate
done
clear
C)
NOT gate
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 72) For a transistor a = 0.9, the value of p is:
A)
1
done
clear
B)
0.09
done
clear
C)
0.9
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 73) The p-n junction diode works a insulator, if connected :
A)
to AC
done
clear
B)
in forward bias
done
clear
C)
in reverse bias
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 74) When arsenic is added as an impurity to silicon the resulting material is :
A)
n-type conductor
done
clear
B)
n-type semiconductor
done
clear
C)
p-type conductor
done
clear
D)
p-type semiconductor
done
clear
View Answer play_arrow
question_answer 75) Which of the following is not an amorphous substance?
A)
Glass
done
clear
B)
Polymers
done
clear
C)
Copper
done
clear
D)
Rubber
done
clear
View Answer play_arrow
question_answer 76) The radiation with maximum penetration power is:
A)
\[\alpha -\]rays
done
clear
B)
\[\beta -\]rays
done
clear
C)
\[\gamma -\]rays
done
clear
D)
cathode rays
done
clear
View Answer play_arrow
question_answer 77) \[{{N}_{2}}\]and CO are:
A)
isomers
done
clear
B)
isoelectronic
done
clear
C)
isotopes
done
clear
D)
isobars
done
clear
View Answer play_arrow
question_answer 78) Which of the following is the strongest Lewis acid?
A)
\[B{{F}_{3}}\]
done
clear
B)
\[BC{{l}_{3}}\]
done
clear
C)
\[BB{{r}_{3}}\]
done
clear
D)
\[B{{I}_{3}}\]
done
clear
View Answer play_arrow
question_answer 79) Which of the following is diamagnetic?
A)
\[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
B)
\[{{[Cu{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
C)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
D)
\[{{[Co{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 80) Non-Ianthanide element is:
A)
\[La\]
done
clear
B)
\[Lu\]
done
clear
C)
\[Pr\]
done
clear
D)
\[Pm\]
done
clear
View Answer play_arrow
question_answer 81) Which of the following is a Lewis base?
A)
\[C{{H}_{4}}\]
done
clear
B)
\[AlC{{l}_{3}}\]
done
clear
C)
\[Al{{(OH)}_{3}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 82) In which of the solutions containing following solutes, its normality is equal to its molarity?
A)
Boric acid
done
clear
B)
Sodium carbonate
done
clear
C)
Sulphuric acid
done
clear
D)
Phosphoric acid
done
clear
View Answer play_arrow
question_answer 83) Enthalpy of\[N{{H}_{3}}\]is\[-\,46\text{ }kJ\text{ }mo{{l}^{-1}},\text{ }\Delta H\]for the reaction:\[2N{{H}_{3}}(g)\to {{N}_{2}}(g)+3{{H}_{2}}(g)\]is:
A)
46 kJ
done
clear
B)
92 kJ
done
clear
C)
\[-\,92\text{ }kJ\]
done
clear
D)
\[-\,23\text{ }kJ\]
done
clear
View Answer play_arrow
question_answer 84) Which one of the following in presence of sunlight will react with chlorine to give benzyl chloride?
A)
Benzene
done
clear
B)
Toluene
done
clear
C)
Benzoicacid
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 85) Which of the following is formed when pent-1-ene reacts with\[HCl\]?
A)
3-chloropentane
done
clear
B)
2-chloropentane
done
clear
C)
1,2-dichloropentane
done
clear
D)
1-chloropentane
done
clear
View Answer play_arrow
question_answer 86) The reaction of formaldehyde with Grignard reagent followed by hydrolysis yields:
A)
a primary alcohol
done
clear
B)
a secondary alcohol
done
clear
C)
a tertiary alcohol
done
clear
D)
a phenol
done
clear
View Answer play_arrow
question_answer 87) Which is the correct order of decreasing strength?
A)
\[ClC{{H}_{2}}COOH>C{{H}_{3}}COOH>HCOOH>\]\[{{C}_{6}}{{H}_{5}}OH\]
done
clear
B)
\[ClC{{H}_{2}}COOH>HCOOH>C{{H}_{3}}COOH>\]\[{{C}_{6}}{{H}_{5}}OH\]
done
clear
C)
\[ClC{{H}_{2}}COOH>HCOOH>{{C}_{6}}{{H}_{5}}OH>\]\[C{{H}_{3}}COOH\]
done
clear
D)
\[ClC{{H}_{2}}COOH>C{{H}_{3}}COOH>{{C}_{6}}{{H}_{5}}OH>\]\[HCOOH\]
done
clear
View Answer play_arrow
question_answer 88) Which of the following is the strongest base?
A)
Methylamine
done
clear
B)
Ammonia
done
clear
C)
Dimethylamine
done
clear
D)
Triethyl amine
done
clear
View Answer play_arrow
question_answer 89) Which of the following does not react with ammoniacal\[AgN{{O}_{3}}\]but decolourises bromine water?
A)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{8}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 90) The general molecular formula for a cycloalkyl group is:
A)
\[{{C}_{n}}{{H}_{2n+1}}\]
done
clear
B)
\[{{C}_{n}}{{H}_{2n-1}}\]
done
clear
C)
\[{{C}_{n}}{{H}_{2n}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 91) Which of the following is the true value of the gas constant R in joule/degree/mol?
A)
8.314
done
clear
B)
\[6.023\times {{10}^{23}}\]
done
clear
C)
\[8.314\times {{10}^{7}}\]
done
clear
D)
6.023
done
clear
View Answer play_arrow
question_answer 92) Which of the following hybridization corresponds to trigonal bipyramidal geometry?
A)
\[s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{2}}d\]
done
clear
D)
\[s{{p}^{3}}d\]
done
clear
View Answer play_arrow
question_answer 93) Which of the following ligands can yield linkage isomerism?
A)
\[{{[C{{O}_{3}}]}^{2-}}\]
done
clear
B)
\[{{[N{{O}_{3}}]}^{-}}\]
done
clear
C)
\[{{[S{{O}_{3}}]}^{2-}}\]
done
clear
D)
\[{{[SCN]}^{-}}\]
done
clear
View Answer play_arrow
question_answer 94) \[r=k[C][D]\]for the reaction\[C+D\to \]products. If D is taken in excess, the order of reaction would be:
A)
1
done
clear
B)
0
done
clear
C)
2
done
clear
D)
unpredictable
done
clear
View Answer play_arrow
question_answer 95) The rate constant of a first order reaction, where half-life is 8 min, is:
A)
\[1.44{{s}^{-1}}\]
done
clear
B)
\[0.72\times {{10}^{-3}}{{s}^{-1}}\]
done
clear
C)
\[1.44\times {{10}^{-3}}{{s}^{-1}}\]
done
clear
D)
\[2.88\times {{10}^{-3}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 96) Choose the false statement from the following:
A)
The order and the molecularity of an elementary reaction are equal
done
clear
B)
The rate constant of a reaction varies with temperature
done
clear
C)
The order of a reaction is a theoretical concept
done
clear
D)
The hydrolysis of an ester in basic medium is second order reaction
done
clear
View Answer play_arrow
question_answer 97) Which of the following is not an ideal solution?
A)
\[CC{{l}_{4}}\]and\[SiC{{l}_{4}}\]
done
clear
B)
\[{{H}_{2}}O\]and \[{{C}_{4}}{{H}_{9}}OH\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}Br\]and\[{{C}_{2}}{{H}_{5}}I\]
done
clear
D)
n-hexane and n-heptane
done
clear
View Answer play_arrow
question_answer 98) At a given temperature isotonic solutions have the same:
A)
density
done
clear
B)
volume
done
clear
C)
normality
done
clear
D)
molar concentration
done
clear
View Answer play_arrow
question_answer 99) On denaturation of protein:
A)
its primary structure changes
done
clear
B)
its secondary/tertiary structure changes
done
clear
C)
hydrolysis takes place
done
clear
D)
none of the above happen
done
clear
View Answer play_arrow
question_answer 100) The reaction of chloroform with ethylamine in alcoholic alkali gives:
A)
\[{{C}_{2}}{{H}_{5}}CN\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}Cl\]
done
clear
C)
\[{{C}_{3}}{{H}_{7}}Cl\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}NC\]
done
clear
View Answer play_arrow
question_answer 101) Butanenitrile is prepared by heating:
A)
propyl alcohol with KCN
done
clear
B)
butyl alcohol with KCN
done
clear
C)
butyl chloride with KCN
done
clear
D)
propyl chloride with KCN
done
clear
View Answer play_arrow
question_answer 102) Which one of the following is produced by reduction of RCN in sodium ethanol?
A)
\[RCON{{H}_{2}}\]
done
clear
B)
\[RCOON{{H}_{4}}\]
done
clear
C)
\[RC{{H}_{2}}N{{H}_{2}}\]
done
clear
D)
\[{{(RC{{H}_{2}})}_{3}}N\]
done
clear
View Answer play_arrow
question_answer 103) Which one of the following on reaction with excess of\[MeMgI\]would give a tertiary alcohol?
A)
Methyl acetate
done
clear
B)
Benzaldehyde
done
clear
C)
Ethyl formate
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 104) Weight-of 4 L of\[{{N}_{2}}\]gas at NTP is:
A)
56 g
done
clear
B)
2.5 g
done
clear
C)
5 g
done
clear
D)
28 g
done
clear
View Answer play_arrow
question_answer 105) Purification of the muddy water by alum takes place through:
A)
dialysis
done
clear
B)
electrophoresis
done
clear
C)
coagulation
done
clear
D)
filtration
done
clear
View Answer play_arrow
question_answer 106) A colloidal system in which a liquid is dispersed in a liquid is called:
A)
precipitate
done
clear
B)
emulsion
done
clear
C)
gel
done
clear
D)
sol
done
clear
View Answer play_arrow
question_answer 107) Electrophoresis is the movement of colloidal particles under the effect of:
A)
light
done
clear
B)
gravity
done
clear
C)
magnetic field
done
clear
D)
electric field
done
clear
View Answer play_arrow
question_answer 108) IUPAC name of\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CH(C{{H}_{3}})COC{{H}_{3}}\]is:
A)
isohexanone
done
clear
B)
heptanone
done
clear
C)
hexanone-5
done
clear
D)
3-methylhexanone-2
done
clear
View Answer play_arrow
question_answer 109) Which one of the following has an aromatic character?
A)
Cyclopentadiene
done
clear
B)
Cycloheptatrienylium cation
done
clear
C)
Cyclopropyne
done
clear
D)
Cycloheptatrienyl anion
done
clear
View Answer play_arrow
question_answer 110) In the presence of Lewis acid toluene reacts with chlorine to give:
A)
m-chlorotoluene
done
clear
B)
o/p-chlorotoluene
done
clear
C)
benzylchloride
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 111) But-2-ene could exist as:
A)
E and Z isomers
done
clear
B)
R and S isomers
done
clear
C)
geometrical isomers
done
clear
D)
only one compound
done
clear
View Answer play_arrow
question_answer 112) Which of the following on addition of water in presence of acid gives a ketone?
A)
Propene
done
clear
B)
Butene-2
done
clear
C)
Propyne
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 113) Phenol on treatment with bromine water in the absence of a Lewis acid gives:
A)
o/p-bromophenol
done
clear
B)
m-bromophenol
done
clear
C)
tribromophenol
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 114) Which of the following on oxidation with\[Cr{{O}_{3}}\]would give a product that would give iodoform test?
A)
Butanol-2
done
clear
B)
Methanol
done
clear
C)
t-butanol
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 115) The reaction of chloroform and sodium hydroxide with phenol to form salicylaldehyde is called:
A)
aldol condensation
done
clear
B)
Cannizaro reaction
done
clear
C)
Claisen reaction
done
clear
D)
Reimer-Tiemann reaction
done
clear
View Answer play_arrow
question_answer 116) Which one of the following can be converted to\[C{{H}_{3}}CH=CHCHO\]?
A)
Acetone
done
clear
B)
Acetaldehyde
done
clear
C)
Propanaldehyde
done
clear
D)
Formaldehyde
done
clear
View Answer play_arrow
question_answer 117) Phenylethyl ether when boiled with concentrated\[HBr\]gives:
A)
phenol and ethyl bromide
done
clear
B)
p-bromophenol and ethyl bromide
done
clear
C)
p-bromophenylethyl ether
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 118) Which of the following non-metal possesses the atomicity half that of sulphur?
A)
Nitrogen
done
clear
B)
Oxygen
done
clear
C)
Phosphorus
done
clear
D)
Arsenic
done
clear
View Answer play_arrow
question_answer 119) Which of the following has maximum unpaired electrons?
A)
Scandium
done
clear
B)
Chromium
done
clear
C)
Manganese
done
clear
D)
Iron
done
clear
View Answer play_arrow
question_answer 120) Which of the following compounds is square planar and does not have any unpaired electron?
A)
\[Ni{{(CO)}_{4}}\]
done
clear
B)
\[{{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
C)
\[{{[NiC{{l}_{4}}]}^{2-}}\]
done
clear
D)
\[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 121) Which is insoluble in water?
A)
Sodium carbonate
done
clear
B)
Sodium bicarbonate
done
clear
C)
Calcium carbonate
done
clear
D)
Calcium bicarbonate
done
clear
View Answer play_arrow
question_answer 122) Which of the following is magnetite?
A)
\[FeC{{O}_{3}}\]
done
clear
B)
\[F{{e}_{2}}{{O}_{3}}\]
done
clear
C)
\[F{{e}_{3}}{{O}_{4}}\]
done
clear
D)
\[F{{e}_{2}}{{O}_{3}}.3{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 123) Which of the following is the smallest anion?
A)
\[{{O}^{2-}}\]
done
clear
B)
\[{{S}^{2-}}\]
done
clear
C)
\[C{{l}^{-}}\]
done
clear
D)
\[B{{r}^{-}}\]
done
clear
View Answer play_arrow
question_answer 124) In which of the following compounds manganese has oxidation number equal to that of iodine in\[KI{{O}_{4}}\]?
A)
Potassium manganite
done
clear
B)
Potassium permanganate
done
clear
C)
Manganous chloride
done
clear
D)
Manganese chloride
done
clear
View Answer play_arrow
question_answer 125) Which of the following compounds is expected to yield a white precipitate with \[AgN{{O}_{3}}\]solution?
A)
\[CHC{{l}_{3}}\]
done
clear
B)
\[CC{{l}_{4}}\]
done
clear
C)
\[[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]\]
done
clear
D)
\[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl\]
done
clear
View Answer play_arrow
question_answer 126) How much\[FeS{{O}_{4}}.7{{H}_{2}}O\]will be oxidised by an acidified solution containing\[9.48\text{ }g\text{ }KMn{{O}_{4}}\]?
A)
83.4 g
done
clear
B)
16.7 g
done
clear
C)
1.67 g
done
clear
D)
8.3 g
done
clear
View Answer play_arrow
question_answer 127) The oxidation number of sulphur is\[-1\]in:
A)
\[FeS\]
done
clear
B)
\[Fe{{S}_{2}}\]
done
clear
C)
\[NaO-\underset{\begin{smallmatrix} | \\ ONa \end{smallmatrix}}{\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{S}}}\,=O\]
done
clear
D)
\[\underset{\begin{smallmatrix} | \\ ONa \end{smallmatrix}}{\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{S}}}\,-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{S}}\,-ONa\]
done
clear
View Answer play_arrow
question_answer 128) The extraction of metal from ore is called:
A)
refining
done
clear
B)
metallurgy
done
clear
C)
leaching
done
clear
D)
bleaching
done
clear
View Answer play_arrow
question_answer 129) Calculate\[{{t}_{1/2}}\]for a radioactive species having \[A=2.09\times {{10}^{-4}}mi{{n}^{-1}}\]:
A)
23 days
done
clear
B)
230 days
done
clear
C)
0.23 days
done
clear
D)
2.3 days
done
clear
View Answer play_arrow
question_answer 130) Which of the following represents Zeises salt?
A)
\[{{K}_{2}}[PtC{{l}_{4}}({{\eta }^{2}}-{{C}_{2}}{{H}_{4}})]\]
done
clear
B)
\[K[PtC{{l}_{4}}{{({{\eta }^{2}}-{{C}_{2}}{{H}_{4}})}_{2}}]\]
done
clear
C)
\[K[PtC{{l}_{3}}({{\eta }^{2}}-{{C}_{2}}{{H}_{4}})]\]
done
clear
D)
\[{{K}_{2}}[PtC{{l}_{4}}{{({{\eta }^{2}}-{{C}_{2}}{{H}_{4}})}_{2}}]\]
done
clear
View Answer play_arrow
question_answer 131) Which of the following atomic models involve the concept of stationary orbitals?
A)
Bohrs model
done
clear
B)
Thomson model
done
clear
C)
Rutherford model
done
clear
D)
Wave model
done
clear
View Answer play_arrow
question_answer 132) How much\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}-5{{H}_{2}}O\]is required for making 100 mL of its 0.5 N solution?
A)
12.4 g
done
clear
B)
6.2.g
done
clear
C)
124 g
done
clear
D)
62 g
done
clear
View Answer play_arrow
question_answer 133) Which of the following compounds has colour but no unpaired electrons?
A)
\[KMn{{O}_{4}}\]
done
clear
B)
\[{{K}_{2}}Mn{{O}_{4}}\]
done
clear
C)
\[MnS{{O}_{4}}\]
done
clear
D)
\[MnC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 134) Which of the following is metaphosphoric acid?
A)
\[{{H}_{3}}{{P}_{3}}{{O}_{9}}\]
done
clear
B)
\[{{H}_{5}}{{P}_{3}}{{O}_{10}}\]
done
clear
C)
\[{{H}_{9}}{{P}_{2}}{{O}_{7}}\]
done
clear
D)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 135) Which of the following has lowest thermal stability and maximum acid strength?
A)
\[{{H}_{2}}S\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[{{H}_{2}}Se\]
done
clear
D)
\[{{H}_{2}}Te\]
done
clear
View Answer play_arrow
question_answer 136) Which of the following molecules have unpaired electrons in antibonding molecular orbitals?
A)
\[{{O}_{2}}\]
done
clear
B)
\[{{N}_{2}}\]
done
clear
C)
\[{{C}_{2}}\]
done
clear
D)
\[{{B}_{2}}\]
done
clear
View Answer play_arrow
question_answer 137) In which of the following lanthanides, oxidation state +2 is most stable?
A)
\[Ce\]
done
clear
B)
\[Eu\]
done
clear
C)
\[Tb\]
done
clear
D)
\[Dy\]
done
clear
View Answer play_arrow
question_answer 138) The species containing shortest\[OO\]bond length is:
A)
\[{{O}_{2}}\]
done
clear
B)
\[O_{2}^{-}\]
done
clear
C)
\[O_{2}^{2-}\]
done
clear
D)
\[O_{2}^{+}\]
done
clear
View Answer play_arrow
question_answer 139) In the reaction\[{{O}_{2}}(g)+{{N}_{2}}(g)2NO(g);\]\[\Delta H=80kJ\]. The decomposition of NO is favoured by:
A)
increasing concentration of\[{{N}_{2}}\]
done
clear
B)
increase in pressure
done
clear
C)
increase in temperature
done
clear
D)
decrease in temperature
done
clear
View Answer play_arrow
question_answer 140) If\[[{{H}^{+}}]\]of a solution of\[pH=6\]is decreased to 100 times, the solution will be:
A)
more acidic
done
clear
B)
basic
done
clear
C)
neutral
done
clear
D)
of the same pH
done
clear
View Answer play_arrow
question_answer 141) Which of the following species is amphoteric in nature?
A)
\[{{H}_{3}}{{O}^{+}}\]
done
clear
B)
\[HSO_{3}^{-}\]
done
clear
C)
\[SO_{3}^{2-}\]
done
clear
D)
\[SO_{4}^{2-}\]
done
clear
View Answer play_arrow
question_answer 142) Which of the following salts has value of the vant Hoffs factor equal to that of \[{{K}_{3}}[Fe{{(CN)}_{6}}]\]?
A)
\[A{{l}_{2}}{{(S{{O}_{4}})}_{3}}\]
done
clear
B)
\[NaCl\]
done
clear
C)
\[Al{{(N{{O}_{3}})}_{3}}\]
done
clear
D)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 143) The factor which does not affect the heat of reaction is:
A)
physical state of the reactants and products
done
clear
B)
temperature of the reaction
done
clear
C)
whether the reaction is carried out in an open vessel or a closed vessel
done
clear
D)
whether the reaction is carried out directly or indirectly
done
clear
View Answer play_arrow
question_answer 144) Which of the following is a disproportionation reaction?
A)
\[2C{{u}^{+}}\xrightarrow[{}]{{}}C{{u}^{2+}}+Cu\]
done
clear
B)
\[Cu\xrightarrow[{}]{-e}C{{u}^{+}}\]
done
clear
C)
\[C{{l}_{2}}\xrightarrow[{}]{+2e}C{{l}^{-}}\]
done
clear
D)
\[2Mg+{{O}_{2}}\xrightarrow{{}}2MgO\]
done
clear
View Answer play_arrow
question_answer 145) For the process\[{{H}_{2}}O(l){{H}_{2}}O(g)\]at\[{{100}^{o}}C,\] atm pressure:
A)
\[\Delta H=T\Delta S\]
done
clear
B)
\[\Delta H=0\]
done
clear
C)
\[\Delta H=\Delta E\]
done
clear
D)
\[\Delta E=0\]
done
clear
View Answer play_arrow
question_answer 146) A tightly closed dessicator in action is an example of:
A)
open system
done
clear
B)
isolated system
done
clear
C)
closed system
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 147) Which of the following metals can displace any of the remaining three from their respective salt solution?
A)
Ag
done
clear
B)
Cu
done
clear
C)
Zn
done
clear
D)
Fe
done
clear
View Answer play_arrow
question_answer 148) On electrolysis of dilute\[{{H}_{2}}S{{O}_{4}}\]using platinum electrodes, the gas evolved at the anode is:
A)
\[{{O}_{2}}\]
done
clear
B)
\[{{H}_{2}}\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[S{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 149) Which of the following is a strong electrolyte?
A)
\[HCN\]
done
clear
B)
\[Ca{{(N{{O}_{3}})}_{2}}\]
done
clear
C)
\[{{H}_{2}}S{{O}_{3}}\]
done
clear
D)
\[N{{H}_{4}}OH\]
done
clear
View Answer play_arrow
question_answer 150) The electrolysis of\[AlC{{l}_{3}}\]deposits 22.5 g of the metal. The number of Faradays passed must be:
A)
1.0
done
clear
B)
1.5
done
clear
C)
2.0
done
clear
D)
2.5
done
clear
View Answer play_arrow
question_answer 151) Cellulose is :
A)
hexose polysaccharide
done
clear
B)
heptose polysaccharide
done
clear
C)
hetero polysaccharide
done
clear
D)
pentosan polysaccharide
done
clear
View Answer play_arrow
question_answer 152) Purines in RNA are :
A)
adenine and guanine
done
clear
B)
thiamine and cytosine
done
clear
C)
thymine and uracil
done
clear
D)
uracil and cytosine
done
clear
View Answer play_arrow
question_answer 153) The model of DNA was proposed by :
A)
Watson and Crick
done
clear
B)
Roebert Hooke
done
clear
C)
Schleiden and Schwann
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 154) DNA and RNA are different molecules as :
A)
DNA has cytosine and RNA has guanine
done
clear
B)
DNA has uracil and RNA has thiamine
done
clear
C)
DNA is a micromolecule and RNA is a macromolecule
done
clear
D)
DNA has thymine and RNA has uracil
done
clear
View Answer play_arrow
question_answer 155) A nucleoside differs from nucleotide in not having the :
A)
sugar
done
clear
B)
nitrogen base
done
clear
C)
glucose
done
clear
D)
phosphate group
done
clear
View Answer play_arrow
question_answer 156) Nucleic acid can be fragmented by the enzyme:
A)
polymerases
done
clear
B)
nucleases
done
clear
C)
proteases
done
clear
D)
ligases
done
clear
View Answer play_arrow
question_answer 157) Prokaryotic cells occur in :
A)
PPLO
done
clear
B)
bacteria
done
clear
C)
cyanobacteria
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 158) Prokaryotic ribosomes are:
A)
30S
done
clear
B)
50S
done
clear
C)
70S
done
clear
D)
80S
done
clear
View Answer play_arrow
question_answer 159) Flagella with single strand and composed of flagellin is found in:
A)
prokaryotes
done
clear
B)
eukaryotes
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 160) Animal cell differs from plant cells in not having the :
A)
Cell wall
done
clear
B)
plastids
done
clear
C)
glyoxysomes
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 161) Which one of the following is absent from the plant cell?
A)
Cell wall
done
clear
B)
Dictyosomes
done
clear
C)
Centrioles
done
clear
D)
Plastids
done
clear
View Answer play_arrow
question_answer 162) Mitochondria and chloroplast are regarded : endosymbionts because :
A)
they do not have de-novo origin
done
clear
B)
they both possess nucleic acids
done
clear
C)
their membranes are like those : prokaryotes
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 163) The Ipomea batatas (sweet potato) is :
A)
underground root
done
clear
B)
underground stem
done
clear
C)
modified leaf
done
clear
D)
modified phylloclade
done
clear
View Answer play_arrow
question_answer 164) Phylloclades are :
A)
modified succulent stems
done
clear
B)
modified succulent leaves
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 165) Cork cambium is :
A)
apical meristem
done
clear
B)
lateral meristem
done
clear
C)
promeristem
done
clear
D)
intercalary meristem
done
clear
View Answer play_arrow
question_answer 166) Vessels and fibres are found in :
A)
xylem of angiosperms
done
clear
B)
xylem of gymnosperms
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 167) Conducting part of phloem according to Haberlandt (1914) is :
A)
hadrom
done
clear
B)
leptom
done
clear
C)
sterom
done
clear
D)
bark
done
clear
View Answer play_arrow
question_answer 168) Secondary xylem is :
A)
wood
done
clear
B)
bark
done
clear
C)
cork
done
clear
D)
best
done
clear
View Answer play_arrow
question_answer 169) Callus is :
A)
material used for healing of injuries in phloem
done
clear
B)
an undifferentiated mass of cells
done
clear
C)
secondary tissue developed by woody plants over a wound
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 170) The most widely accepted theory of ascent of sap is :
A)
capillarity
done
clear
B)
pulsatory movement of living tissue
done
clear
C)
role of atmospheric pressure
done
clear
D)
transpiration pull and cohesion theory
done
clear
View Answer play_arrow
question_answer 171) Which of the following is most important factor in regulation of transpiration?
A)
Humidity
done
clear
B)
Light
done
clear
C)
Temperature
done
clear
D)
Wind
done
clear
View Answer play_arrow
question_answer 172) Which is true for fully turgid cell?
A)
OP = 0
done
clear
B)
DPD = 0
done
clear
C)
OP = DPD
done
clear
D)
TP = 0
done
clear
View Answer play_arrow
question_answer 173) Salt respiration is :
A)
secretion of salt through respiratory channels
done
clear
B)
decrease in respiration during salt absorption
done
clear
C)
active increase in respiration during mineral absorption
done
clear
D)
linking of ion movement with respiratory chain
done
clear
View Answer play_arrow
question_answer 174) Bending of tentacles of Drosera over the insects is :
A)
thigmonasty
done
clear
B)
thigmotropism
done
clear
C)
chemotropism
done
clear
D)
phototropism
done
clear
View Answer play_arrow
question_answer 175) First natural cytokinin, zeatin was discovered by:
A)
Letham
done
clear
B)
Skoog and Miller
done
clear
C)
Bensen
done
clear
D)
Thinman and Went
done
clear
View Answer play_arrow
question_answer 176) First transitory chemical formed by reaction between \[C{{O}_{2}}\]and RuBP is :
A)
PGAL/GAP
done
clear
B)
2 carboxy, 3 keto, 1-5 phosphoribotol
done
clear
C)
PGA
done
clear
D)
dihydroxy acetone phosphate
done
clear
View Answer play_arrow
question_answer 177) The zygospore in Rhizopus develops into :
A)
zygospore
done
clear
B)
progametangium
done
clear
C)
promycelium
done
clear
D)
gametangium
done
clear
View Answer play_arrow
question_answer 178) Cell wall of Chlamydomonas contains :
A)
cellulose
done
clear
B)
glycoproteins
done
clear
C)
protein
done
clear
D)
hemicelluloses
done
clear
View Answer play_arrow
question_answer 179) The rhizoids of Funaria are :
A)
Green branched thread like structures
done
clear
B)
unbranched root
done
clear
C)
branched and multicellular with oblique septa
done
clear
D)
unicellular growth structure
done
clear
View Answer play_arrow
question_answer 180) In Dryopteria:
A)
sporophyte is independent
done
clear
B)
gametophyte is independent
done
clear
C)
both sporophyte and gametophyte are dependent
done
clear
D)
sporophyte is dependent upon gametophyte for its nutrition
done
clear
View Answer play_arrow
question_answer 181) Seed of Pinus possesses:
A)
parent sporophyte as seed coat, perisperm etc.
done
clear
B)
gametophyte like endosperm
done
clear
C)
future sporophyte like embryo
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 182) A flower which can be divided into two equal parts by only one plane is :
A)
actinomorphic
done
clear
B)
zygomorphic
done
clear
C)
regular
done
clear
D)
perfect
done
clear
View Answer play_arrow
question_answer 183) Inflorescence with universal sessile flower is :
A)
spike
done
clear
B)
spikelet
done
clear
C)
catkin
done
clear
D)
spadix
done
clear
View Answer play_arrow
question_answer 184) Contrivances for self-pollination are :
A)
bisexuality
done
clear
B)
homogamy
done
clear
C)
cleistogamy
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 185) Berries, drupes and pomes are :
A)
simple dry fruits
done
clear
B)
simple succulent fruits
done
clear
C)
aggregate fruits
done
clear
D)
composite fruits
done
clear
View Answer play_arrow
question_answer 186) Explants are :
A)
exploited part of a plant
done
clear
B)
a small part grown for tissue culture
done
clear
C)
uprooted plant part for transpiration
done
clear
D)
a plant collected after harvest
done
clear
View Answer play_arrow
question_answer 187) Nitrogen fixers in Azolla are :
A)
Nostoc
done
clear
B)
Anabaena
done
clear
C)
Aulosira
done
clear
D)
Azospirillum
done
clear
View Answer play_arrow
question_answer 188) Bio fertilizers include :
A)
nitrogen fixing bacteria
done
clear
B)
nitrogen fixing cyanobacteria
done
clear
C)
mycorrhiza
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 189) The full form of IPGRI is :
A)
Indian Plant Genetic Resource Institute
done
clear
B)
International Plant Genetic Resource Institute
done
clear
C)
International Potato Genetic Resource Institute
done
clear
D)
International Pine Genetic Resource Institute
done
clear
View Answer play_arrow
question_answer 190) Recombination of genes occur at:
A)
prophase in mitosis
done
clear
B)
prophase-I in meiosis
done
clear
C)
prophase-II in meiosis
done
clear
D)
metaphase-H in meiosis
done
clear
View Answer play_arrow
question_answer 191) Polydactyly in man is due to :
A)
autosomal dominant gene
done
clear
B)
autosomal recessive gene
done
clear
C)
sex-linked dominant gene
done
clear
D)
sex-linked recessive gene
done
clear
View Answer play_arrow
question_answer 192) Variation in character are brought about by :
A)
duplication of chromosomes during mitosis
done
clear
B)
mutations
done
clear
C)
crossing over during meiosis
done
clear
D)
both (a) and (b)
done
clear
View Answer play_arrow
question_answer 193) At which stage of the meiosis, the bivalents are formed?
A)
Leptotene
done
clear
B)
Pachytene
done
clear
C)
Zygotene
done
clear
D)
Diplotene
done
clear
View Answer play_arrow
question_answer 194) Mutation at the chromosomal level, with an addition of individual chromosome is referred to as:
A)
polyploidy
done
clear
B)
polysomy
done
clear
C)
structural mutation
done
clear
D)
point mutation
done
clear
View Answer play_arrow
question_answer 195) A sequence of three bases code along the DNA molecule is called :
A)
genetic code
done
clear
B)
gene pool
done
clear
C)
genetic drift
done
clear
D)
genome
done
clear
View Answer play_arrow
question_answer 196) Endocrine glands :
A)
do not posses ducts
done
clear
B)
sometimes do not have ducts
done
clear
C)
pour their secretion into blood through ducts
done
clear
D)
always have ducts
done
clear
View Answer play_arrow
question_answer 197) Serum is :
A)
plasma
done
clear
B)
plasma minus fibrinogen
done
clear
C)
plasma minus \[C{{a}^{+}}\] ions
done
clear
D)
plasma minus gamma globulins
done
clear
View Answer play_arrow
question_answer 198) Which of the following process does not take place when glucose is eaten?
A)
Digestion
done
clear
B)
Ingestion
done
clear
C)
Assimilation
done
clear
D)
Absorption
done
clear
View Answer play_arrow
question_answer 199) The intestine and stomach in mammals are lined by :
A)
cuboidal epithelium
done
clear
B)
columnar epithelium
done
clear
C)
squamous epithelium
done
clear
D)
stratified epithelium
done
clear
View Answer play_arrow
question_answer 200) Uric acid is formed in human from :
A)
purines
done
clear
B)
proteins
done
clear
C)
glucose
done
clear
D)
pyrimidines
done
clear
View Answer play_arrow
question_answer 201) The delicious food generally makes mouir watery. It is due to :
A)
hormonal response
done
clear
B)
neural response
done
clear
C)
olfactory response
done
clear
D)
optic response
done
clear
View Answer play_arrow
question_answer 202) Night blindness is a deficiency disease due : vitamin :
A)
A
done
clear
B)
\[{{B}_{12}}\]
done
clear
C)
C
done
clear
D)
K
done
clear
View Answer play_arrow
question_answer 203) Ligaments :
A)
bind one organ to another
done
clear
B)
attach muscles to bone
done
clear
C)
attach bone to bone
done
clear
D)
both (a) and (b)
done
clear
View Answer play_arrow
question_answer 204) The thinnest skin is present on the :
A)
eyelids
done
clear
B)
soles of feet
done
clear
C)
back of the hand
done
clear
D)
forehead
done
clear
View Answer play_arrow
question_answer 205) Mammary glared is enlarged and modified :
A)
sweat glands
done
clear
B)
sebaceous tissue
done
clear
C)
adipose tissue
done
clear
D)
hair bulb
done
clear
View Answer play_arrow
question_answer 206) Bile is secreted by :
A)
liver
done
clear
B)
gall bladder
done
clear
C)
pancreas
done
clear
D)
duodenal wall
done
clear
View Answer play_arrow
question_answer 207) What distinguishes an insect from crustacean?
A)
Number of eyes
done
clear
B)
Arrangement of nerve cords
done
clear
C)
Number of appendages
done
clear
D)
Presence of wings
done
clear
View Answer play_arrow
question_answer 208) The joint of the hip and shoulders are called :
A)
hinge joint
done
clear
B)
ball and socket joint
done
clear
C)
pivot joint
done
clear
D)
ellipsoid joint
done
clear
View Answer play_arrow
question_answer 209) The nature of exoskeleton in echinoderms is :
A)
calcareous
done
clear
B)
chitinous
done
clear
C)
siliceous
done
clear
D)
tunicin
done
clear
View Answer play_arrow
question_answer 210) Which of the following takes food but has no alimentary canal ?
A)
Ascaris
done
clear
B)
leech
done
clear
C)
Taenia
done
clear
D)
liver fluke
done
clear
View Answer play_arrow
question_answer 211) Salamander is :
A)
Mollusca
done
clear
B)
Annelida
done
clear
C)
Bird
done
clear
D)
Amphibian
done
clear
View Answer play_arrow
question_answer 212) Which of the following respires by gills?
A)
Prawn
done
clear
B)
Frog
done
clear
C)
Crocodile
done
clear
D)
Whale
done
clear
View Answer play_arrow
question_answer 213) The dinosaurs were maximum during the period of:
A)
Jurassic
done
clear
B)
Triassic
done
clear
C)
Cretaceous
done
clear
D)
Palaeocene
done
clear
View Answer play_arrow
question_answer 214) The gas that killed large number of people in Bhopal included :
A)
phosgene
done
clear
B)
methyl isocyanide
done
clear
C)
DDT
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 215) Haemophilia is :
A)
sex-linked disease
done
clear
B)
autosomal disease
done
clear
C)
bacterial disease
done
clear
D)
viral disease
done
clear
View Answer play_arrow
question_answer 216) Polio causes :
A)
measles
done
clear
B)
paralysis
done
clear
C)
mumps
done
clear
D)
malaria
done
clear
View Answer play_arrow
question_answer 217) Causative agent of tuberculosis (TB) is :
A)
Salmonella
done
clear
B)
Pneumococcus
done
clear
C)
Mycobacterium
done
clear
D)
Streptococcus
done
clear
View Answer play_arrow
question_answer 218) The universal donor is :
A)
blood group A
done
clear
B)
blood group B
done
clear
C)
blood group AB
done
clear
D)
blood group O
done
clear
View Answer play_arrow
question_answer 219) The Rh factor is concerned with :
A)
monkey
done
clear
B)
gorilla
done
clear
C)
apes
done
clear
D)
pig
done
clear
View Answer play_arrow
question_answer 220) Body cavity lined by mesoderm is :
A)
coelom
done
clear
B)
coelenterons
done
clear
C)
blastocoel
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 221) Charles Darwins evolutionary theory is :
A)
theory of inheritance of acquired characters
done
clear
B)
mutation theory
done
clear
C)
theory of natural selection
done
clear
D)
use and disuse theory
done
clear
View Answer play_arrow
question_answer 222) Fossil evidences of evolution are called :
A)
embryology
done
clear
B)
anatomy
done
clear
C)
paleontology
done
clear
D)
biogeography
done
clear
View Answer play_arrow
question_answer 223) The origin of species from pre-existing species is:
A)
isolation
done
clear
B)
speciation
done
clear
C)
polyploidy
done
clear
D)
mutation
done
clear
View Answer play_arrow
question_answer 224) Wax is obtained from :
A)
jojoba
done
clear
B)
guayie
done
clear
C)
leucaena
done
clear
D)
subabul
done
clear
View Answer play_arrow
question_answer 225) Chitin is found in :
A)
Mollusca
done
clear
B)
Arthropoda
done
clear
C)
Echinodermata
done
clear
D)
Coelenterata
done
clear
View Answer play_arrow