question_answer 1) The equation of state of real gas can be expressed as \[\left( P+\frac{a}{{{V}^{2}}} \right)(V-b)=RT\], where P is the pressure, V is the volume, T is the absolute temperature and a, b and R are constants. What are the dimensions of a?
A)
\[[{{M}^{0}}{{L}^{3}}{{T}^{-2}}]\]
done
clear
B)
\[[{{M}^{0}}{{L}^{3}}{{T}^{0}}]\]
done
clear
C)
\[[M{{L}^{-2}}{{T}^{-2}}]\]
done
clear
D)
\[[M{{L}^{5}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
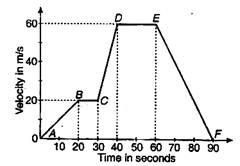
question_answer 2)
The velocity versus time curve of a moving point is as given in the figure. The maximum acceleration is:
A)
\[1\text{ }m/{{s}^{2}}\]
done
clear
B)
\[4m/{{s}^{2}}\]
done
clear
C)
\[2\text{ }m/{{s}^{2}}\]
done
clear
D)
\[1.5\text{ }m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 3) The horizontal range of a projectile is \[4\sqrt{3}\] times the maximum height achieved by it, then the angle of projection is:
A)
\[30{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
\[90{}^\circ \]
done
clear
View Answer play_arrow
question_answer 4) A block slides with a velocity of 10 m/s on a rough horizontal surface. It comes to rest after covering a distance of 50 m. If g is \[10\text{ }m/{{s}^{2}},\] then the coefficient of dynamic friction between the block and surface is:
A)
1
done
clear
B)
10
done
clear
C)
2
done
clear
D)
0.1
done
clear
View Answer play_arrow
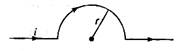
question_answer 5)
A body slides without friction from a height h along a track shown in figure, so that it loops the loop in the vertical plane. If the radius of the loop is R, what should be the minimum value of h in terms of R, so that the body is just able to loop the loop. There is no friction between the body and the track :
A)
h=R
done
clear
B)
h = 2R
done
clear
C)
\[h=\frac{5}{2}R\]
done
clear
D)
h = 4R
done
clear
View Answer play_arrow
question_answer 6) If g is the acceleration due to gravity on the earths surface, the gain in potential energy of an object of mass m raised from the surface of earth to a height equal to the radius R of the earth is :
A)
\[\frac{1}{4}mgR\]
done
clear
B)
mgR
done
clear
C)
2mgR
done
clear
D)
\[\frac{1}{2}\]mgR
done
clear
View Answer play_arrow
question_answer 7) A neutron moving with a velocity v, collides elastically with an atom of mass number A. The collision is head on. If the initial kinetic energy of a neutron is e, then the kinetic energy of neutron after the collision is :
A)
\[{{\left( \frac{1+A}{1-A} \right)}^{2}}E\]
done
clear
B)
\[{{\left( \frac{1-A}{1+A} \right)}^{2}}E\]
done
clear
C)
\[\left( \frac{1+A}{1-A} \right)E\]
done
clear
D)
\[\left( \frac{1-A}{1+A} \right)E\]
done
clear
View Answer play_arrow
question_answer 8)
A uniform thin bar of mass 3 kg and length 0.9 m is bent to make an equilateral triangle. The moment of inertia of the triangle through its centre of mass and perpendicular to plane of triangle is :
A)
\[0.030\text{ }kg-{{m}^{2}}\]
done
clear
B)
\[0.015\text{ }kg-{{m}^{2}}\]
done
clear
C)
\[0.090\text{ }kg-{{m}^{2}}\]
done
clear
D)
\[0.045\text{ }kg-{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 9) In case of a book lying on table :
A)
action of book and reaction of table on book are equal and opposite and are inclined to vertical
done
clear
B)
action and reaction are equal but act in the same direction
done
clear
C)
action and reaction are equal and opposite and act perpendicular to the surface of contact
done
clear
D)
action and reaction are not equal but act in opposite direction
done
clear
View Answer play_arrow
question_answer 10) A particle is subjected to acceleration\[a=\alpha t+\beta {{t}^{2}}\], where \[\alpha \] and\[\beta \] are constants. The position and velocity of the particle att = 0 are x0 and v0 respectively. The expression for position of particle at time c is :
A)
\[x(t)={{x}_{0}}+{{v}_{0}}t+\frac{1}{6}\alpha {{t}^{3}}+\frac{1}{12}\beta {{t}^{4}}\]
done
clear
B)
\[x(t)={{x}_{0}}+{{v}_{0}}t+\frac{1}{6}\alpha {{t}^{2}}+\frac{1}{24}\beta {{t}^{3}}\]
done
clear
C)
\[x(t)={{x}_{0}}+{{v}_{0}}t+\frac{1}{12}\alpha {{t}^{2}}+\frac{1}{6}\beta {{t}^{3}}\]
done
clear
D)
\[x(t)={{x}_{0}}+{{v}_{0}}t+\frac{1}{6}\alpha {{t}^{2}}+\frac{1}{12}\beta {{t}^{3}}\]
done
clear
View Answer play_arrow
question_answer 11) The period of revolution of an earth satellite close to the surface of the earth is 90 min. The time period of another earth satellite in an orbit at a distance of three earth radii from its surface will be :
A)
90 min
done
clear
B)
\[90\times \sqrt{8}\text{ }min\]
done
clear
C)
270 min
done
clear
D)
720 min
done
clear
View Answer play_arrow
question_answer 12) Energy per unit volume of stretched wire is :
A)
\[\frac{1}{2}\]\[\times \]stress\[\times \]strain
done
clear
B)
stress\[\times \]strain
done
clear
C)
\[\frac{1}{2}\] \[\times \]load \[\times \]strain
done
clear
D)
load \[\times \]strain
done
clear
View Answer play_arrow
question_answer 13) A number of small drops coalesce together to form a single drop. The temperature of new drop:
A)
increases
done
clear
B)
decreases
done
clear
C)
remains unchanged
done
clear
D)
may increase or decrease depends on size
done
clear
View Answer play_arrow
question_answer 14) Two hail stones with radii in the ratio of 1 : 2 fall from a great height through the atmosphere. Then the ratio of their momenta after they have attained terminal velocity is :
A)
1 :1
done
clear
B)
1 :4
done
clear
C)
1 : 16
done
clear
D)
1 : 32
done
clear
View Answer play_arrow
question_answer 15) The differential equation of a particle executing simple harmonic motion along y-axis is :
A)
\[\frac{{{d}^{2}}y}{d{{t}^{2}}}+{{\omega }^{2}}y=0\]
done
clear
B)
\[\frac{{{d}^{2}}y}{d{{t}^{2}}}+{{\omega }^{2}}{{y}^{2}}=0\]
done
clear
C)
\[\frac{{{d}^{2}}y}{d{{t}^{2}}}-{{\omega }^{2}}y=0\]
done
clear
D)
\[\frac{dy}{dt}+\omega y=0\]
done
clear
View Answer play_arrow
question_answer 16) A body executes simple harmonic motion with amplitude a. At what displacement from the equilibrium position is its energy half kinetic and half potential?
A)
\[\frac{a}{2}\]
done
clear
B)
\[\frac{a}{\sqrt{2}}\]
done
clear
C)
\[\sqrt{2a}\]
done
clear
D)
\[\frac{a}{3}\]
done
clear
View Answer play_arrow
question_answer 17) The equation of a particle executing simple harmonic motion is: \[y=10\sin \left( \frac{\pi }{2}t+\frac{\pi }{6} \right)cms.\] The time period of simple harmonic motion is:
A)
6 s
done
clear
B)
4 s
done
clear
C)
\[\frac{\pi }{3}s\]
done
clear
D)
\[\frac{\pi }{2}s\]
done
clear
View Answer play_arrow
question_answer 18) A sonometer wire, 100 cm in length has a fundamental frequency of 330 Hz. The velocity of propagation of transverse waves along the wire is:
A)
300 m/s
done
clear
B)
660 m/s
done
clear
C)
115 m/s
done
clear
D)
990 m/s
done
clear
View Answer play_arrow
question_answer 19) A water proofing agent changes the angle of contact:
A)
from acute to \[90{}^\circ \]
done
clear
B)
from obtuse to \[90{}^\circ \]
done
clear
C)
from an acute to an obtuse angle
done
clear
D)
from an obtuse to an acute angle
done
clear
View Answer play_arrow
question_answer 20) A tuning fork vibrating with a sonometer having 20 cm wire produces 5 beats/s. The beat frequency does not change when the length of the wire is changed to 21 cm. The frequency of tuning fork is :
A)
200
done
clear
B)
210
done
clear
C)
205
done
clear
D)
215
done
clear
View Answer play_arrow
question_answer 21) If n, is the resonance frequency of a pipe open at both ends and \[{{n}_{2}}\]the resonance frequency of pipe open at one end only. Both are vibrating in the fundamental mode and both pipes are of same length, then :
A)
\[{{n}_{1}}={{n}_{2}}\]
done
clear
B)
\[2{{n}_{1}}={{n}_{2}}\]
done
clear
C)
\[{{n}_{1}}=2{{n}_{2}}\]
done
clear
D)
\[3{{n}_{1}}=4{{n}_{2}}\]
done
clear
View Answer play_arrow
question_answer 22) The frequency of a radar is 780 MHz. The frequency of reflected wave from aeroplane is increased by 2.6 kHz. Velocity of the aeroplane is :
A)
0.25 km/s
done
clear
B)
0.5 km/s
done
clear
C)
1.0 km/s
done
clear
D)
2 km/s
done
clear
View Answer play_arrow
question_answer 23) If the density of hydrogen gas at NTP is \[0.0000893\text{ }g/c{{m}^{3}},\] then the root mean square velocity of molecules of hydrogen molecules at NTP is:
A)
1840 cm/s
done
clear
B)
184.0 cm/s
done
clear
C)
1840 m/s
done
clear
D)
184.0 m/s
done
clear
View Answer play_arrow
question_answer 24) The first law of thermodynamics is concerned with conservation of:
A)
number of molecules
done
clear
B)
number of moles
done
clear
C)
energy
done
clear
D)
temperature
done
clear
View Answer play_arrow
question_answer 25) An ideal gas undergoes an isothermal change in volume with pressure, then :
A)
\[PV=\] constant
done
clear
B)
\[{{(PV)}^{y}}=\] constant
done
clear
C)
\[P{{V}^{y}}=\] constant
done
clear
D)
\[{{P}^{Y}}V=\]constant
done
clear
View Answer play_arrow
question_answer 26) An ideal gas heat engine operates in a Carnots cycle between \[227{}^\circ C\] and \[127{}^\circ C\]. It absorbs \[6.0\times 104cal\] at higher temperature. The amount of heat converted into work is:
A)
\[4.8\times {{10}^{4}}cal\]
done
clear
B)
\[3.5\times {{10}^{4}}cal\]
done
clear
C)
\[1.6\times {{10}^{4}}cal\]
done
clear
D)
\[1.2\times {{10}^{4}}cal\]
done
clear
View Answer play_arrow
question_answer 27) A bucket full of hot water is kept in a room. It cools from \[75{}^\circ C\] to \[70{}^\circ C\] in f, minutes, from \[70{}^\circ C\] to \[65{}^\circ C\] in \[{{t}_{2}}\] minutes and from \[65{}^\circ C\] to \[60{}^\circ C\] int3 minutes. Then:
A)
\[{{t}_{1}}={{t}_{2}}={{t}_{3}}\]
done
clear
B)
\[{{t}_{1}}>{{t}_{2}}>{{t}_{3}}\]
done
clear
C)
\[{{t}_{1}}<{{t}_{2}}<{{t}_{3}}\]
done
clear
D)
\[{{t}_{1}}<{{t}_{2}}>{{t}_{3}}\]
done
clear
View Answer play_arrow
question_answer 28) The wavelength\[{{\lambda }_{m}}\]of maximum intensity of emission of solar radiation is \[{{\lambda }_{m}}\] = 4753 \[\overset{0}{\mathop{A}}\,\] and from moon is \[{{\lambda }_{m}}\]= 14 \[\mu m.\] The surface tempe-rature of sun and moon are : (given \[b=2.898\times {{10}^{-3}}m-K\])
A)
6097 K, 207 K
done
clear
B)
8097 K, 307 K
done
clear
C)
10,000 K. 400 K
done
clear
D)
3000 K, 100 K
done
clear
View Answer play_arrow
question_answer 29) A sphere of 4 cm radius is suspended within hollow sphere of 6 cm radius. The inner sphere is charged to a potential 3 esu, when the outer sphere is earthed. The charge on the inner sphere is :
A)
54 esu
done
clear
B)
27 esu
done
clear
C)
30 esu
done
clear
D)
36 esu
done
clear
View Answer play_arrow
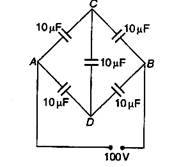
question_answer 30)
Five capacitors each of 10\[\mu F\]capacity are connected to a potential of 100 V as shown in figure. The equivalent capacitance beween ihe points A and B is:
A)
5\[\mu F\]
done
clear
B)
10\[\mu F\]
done
clear
C)
15\[\mu F\]
done
clear
D)
20 \[\mu F\]
done
clear
View Answer play_arrow
question_answer 31) When a potential difference is applied across a conductor, the free electrons in the conductor are set in motion. Two velocities are associated with moving electrons-the drift velocity and average velocity, fact is that the two are :
A)
same
done
clear
B)
same in some conductors and different in other conductors
done
clear
C)
entirely different
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 32) A given piece of wire of length 4 cross-sectional area A has a resistance R. The wire is stretched uniformly to a new length 21 What is the resistance of the wire now?
A)
2R
done
clear
B)
4R
done
clear
C)
8R
done
clear
D)
R
done
clear
View Answer play_arrow
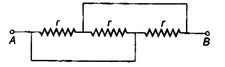
question_answer 33)
Three equal resistors each equal to r are connected as shown in the given figure. The equivalent resistance between points A and B is:
A)
r
done
clear
B)
3r
done
clear
C)
\[\frac{r}{3}\]
done
clear
D)
\[\frac{2}{3}r\]
done
clear
View Answer play_arrow
question_answer 34) Two bulbs of wattage 40 W and 100 W each rated at 200 V are connected in series across a 440 V. What will happen?
A)
40 W bulb will fuse
done
clear
B)
100 W bulb will fuse
done
clear
C)
Both bulbs will fuse
done
clear
D)
Nothing will happen
done
clear
View Answer play_arrow
question_answer 35) An electric bulb rated 100 W at 220 V is operating at 110 V. What is the power consumed?
A)
50 W
done
clear
B)
75 W
done
clear
C)
100 W
done
clear
D)
25 W
done
clear
View Answer play_arrow
question_answer 36)
A straight thin conductor is bent as shown in the adjoining figure. It carries a current i ampere. The radius of the semicircular arc is r metre. The magnetic induction at the centre of semicircular arc is:
A)
\[\frac{{{\mu }_{0}}}{4\pi }\frac{i}{r}tesla\]
done
clear
B)
\[\frac{{{\mu }_{0}}}{4\pi }\frac{i}{r}tesla\]
done
clear
C)
\[\frac{{{\mu }_{0}}}{4\pi }\frac{i}{2r}tesla\]
done
clear
D)
zero
done
clear
View Answer play_arrow
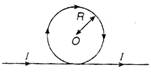
question_answer 37)
An infinitely long conductor is bent into a circle as shown in figure. It carries a current / ampere and the radius of loop is R metre. The magnetic induction at the centre of loop is :
A)
\[\frac{{{\mu }_{0}}}{4\pi }\frac{2I}{R}(\pi +1)tesla\]
done
clear
B)
\[\frac{{{\mu }_{0}}}{4\pi }\frac{2I}{R}(\pi -1)tesla\]
done
clear
C)
\[\frac{{{\mu }_{0}}}{4\pi }\frac{I}{2R}(\pi +1)tesla\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 38) A circular current carrying coil has a radius R. The distance from the centre of the coil on the axis of the coil, where the magnetic induction is\[\frac{1}{8}\] of its value at the centre of coil is :
A)
\[\sqrt{3}R\]
done
clear
B)
\[\frac{R}{\sqrt{3}}\]
done
clear
C)
\[\left( \frac{2}{\sqrt{3}} \right)R\]
done
clear
D)
\[\frac{R}{2\sqrt{3}}\]
done
clear
View Answer play_arrow
question_answer 39) A charge is fired through a magnetic field. The force acting on it is maximum when the direction of motion of the charge and magnetic field is :
A)
zero
done
clear
B)
\[\frac{\pi }{4}\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
\[\pi \]
done
clear
View Answer play_arrow
question_answer 40) Inversion temperature for a thermocouple is the temperature at which thermo emf:
A)
increases
done
clear
B)
remains unchanged
done
clear
C)
changes erratically
done
clear
D)
reverses in sign
done
clear
View Answer play_arrow
question_answer 41) Lenzs law is a consequence of the law of conservation of:
A)
energy
done
clear
B)
charge
done
clear
C)
mass
done
clear
D)
momentum
done
clear
View Answer play_arrow
question_answer 42) A current carrying coil is placed in a unifonr. magnetic field of induction B. If the current in the coil is i, it has N turns and A is the force area of the coil and normal to the surface makes an angle \[\theta \] with B, then the torque experienced by the coil is :
A)
\[BNiA\theta \]
done
clear
B)
\[BNAi\cos \theta \]
done
clear
C)
\[BNAi\sin \theta \]
done
clear
D)
\[BNAi\tan \theta \]
done
clear
View Answer play_arrow
question_answer 43) The current in a self-inductance L = 40 mH increased uniformly from 0 ampere to 10 A in \[4\times {{10}^{-3}}s.\]The induced emf produced in during this process will be :
A)
40 V
done
clear
B)
400 V
done
clear
C)
0.4 V
done
clear
D)
100 V
done
clear
View Answer play_arrow
question_answer 44) The number of turns in the primary and the secondary turns of a transformer are 1000 and 3000 respectively. If 80 V AC is applied to the primary coil of the transformer, then the potential difference per turn of secondary coil is:
A)
0.24 V
done
clear
B)
0.08 V
done
clear
C)
240 V
done
clear
D)
2400 V
done
clear
View Answer play_arrow
question_answer 45) A current \[I={{I}_{0}}\sin \left( \omega t-\frac{\pi }{2} \right)\] flows in an AC potential of \[E={{E}_{0}}sin\] to t has been applied, then the power consumption in the circuit will be:
A)
\[\frac{{{E}_{0}}{{I}_{0}}}{\sqrt{2}}\]
done
clear
B)
\[\frac{EI}{\sqrt{2}}\]
done
clear
C)
\[\frac{{{E}_{0}}{{I}_{0}}}{2}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 46) An LCR series circuit is connected to a source of alternating current. At resonance the applied voltage and current flowing through the circuit will have a phase difference of :
A)
zero
done
clear
B)
\[\frac{\pi }{4}\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
\[\pi \]
done
clear
View Answer play_arrow
question_answer 47) An electric bulb in series with a large inductor when connected across a DC source takes a little time before reaching a stable glow. If an iron core is inserted into the inductor the time delay of reaching stable glow will:
A)
decrease
done
clear
B)
increase
done
clear
C)
remains the same
done
clear
D)
may increase or decrease
done
clear
View Answer play_arrow
question_answer 48) In Millikans oil drop experiment, an oil drop of radius r and charge q is held in equilibrium between plates of a parallel plate capacitor when the potential difference is V. To keep a drop of radius 2 r with charge 2q in equilibrium between the plates, the potential difference required is :
A)
V
done
clear
B)
2 V
done
clear
C)
4 V
done
clear
D)
8 V
done
clear
View Answer play_arrow
question_answer 49) One milli watt of light of wavelength 4560 \[\overset{0}{\mathop{A}}\,\] is incident on a cesium surface of work function 1.9 eV. Given that quantum of efficiency of photoelectric emission is 0.5%, Planck constant \[h=6.62\times {{10}^{-34}}J-s,\] velocity of light \[c=3\times {{10}^{8}}m/s,\]the photoelectric current liberated is :
A)
\[1.84\times {{10}^{-6}}A\]
done
clear
B)
\[1.84\times {{10}^{-7}}A\]
done
clear
C)
\[1.84\times {{10}^{-5}}A\]
done
clear
D)
\[1.84\times {{10}^{-4}}A\]
done
clear
View Answer play_arrow
question_answer 50) Light of frequency 1.5 times the threshold frequency is incident on a photosensitive material photoelectric current is emitted. If the frequency of light is halved and intensity is doubled, the photoelectric current becomes:
A)
4 times the original current
done
clear
B)
2 times the original current
done
clear
C)
half the original current
done
clear
D)
zero times the original current
done
clear
View Answer play_arrow
question_answer 51) Light of wavelength 3500\[\overset{0}{\mathop{A}}\,\] is incident on two metals A and B, A of work function 4.2 eV and B of work function 1.19 eV respectively. The photoelectrons will be emitted by :
A)
metal A
done
clear
B)
metal B
done
clear
C)
both A and B
done
clear
D)
neither metal A nor metal B
done
clear
View Answer play_arrow
question_answer 52) Of the various series of hydrogen spectrum, the one which lies wholly in the ultra violet is:
A)
Brackett series
done
clear
B)
Paschen series
done
clear
C)
Lyman series
done
clear
D)
Balmer series
done
clear
View Answer play_arrow
question_answer 53) The first excitation potential of hydrogen atom in the ground state is :
A)
13.6 V
done
clear
B)
3.4 V
done
clear
C)
10.2 V
done
clear
D)
1.89 V
done
clear
View Answer play_arrow
question_answer 54) According to Bohrs theory of hydrogen atom, the angular momentum of an electron in any orbit of hydrogen atom is:
A)
directly proportional to radius of orbit
done
clear
B)
inversely proportional to the radius of orbit
done
clear
C)
directly proportional to the square of the orbit radius
done
clear
D)
directly proportional to the square root of the radius of the orbit
done
clear
View Answer play_arrow
question_answer 55) If the wavelength of first member of Balmer series in hydrogen spectrum is 6563\[\overset{0}{\mathop{A}}\,\], the wavelength of second member of Balmer series will be :
A)
1215\[\overset{0}{\mathop{A}}\,\]
done
clear
B)
4861\[\overset{0}{\mathop{A}}\,\]
done
clear
C)
6050\[\overset{0}{\mathop{A}}\,\]
done
clear
D)
data given is insufficient to calculate the value
done
clear
View Answer play_arrow
question_answer 56) In the nuclear fission reaction, \[_{2}^{4}He+_{7}^{14}N\to _{p}^{q}x{{+}_{1}}{{H}^{1}}\] the nucleus \[_{p}^{q}X\]is :
A)
nitrogen of mass 16
done
clear
B)
nitrogen of mass 17
done
clear
C)
oxygen of mass 16
done
clear
D)
oxygen of mass 17
done
clear
View Answer play_arrow
question_answer 57) In atomic reactors cadmium rods are used to control the chain reaction. This is because cadmium:
A)
absorbs some neutrons
done
clear
B)
slows down the neutrons
done
clear
C)
speeds up neutrons
done
clear
D)
emits neutrons
done
clear
View Answer play_arrow
question_answer 58) Nuclear forces are :
A)
attractive only
done
clear
B)
repulsive only
done
clear
C)
attractive or repulsive depending upon separation between the nucleons
done
clear
D)
neither attractive nor repulsive
done
clear
View Answer play_arrow
question_answer 59) The binding energy per nucleon for deuteron \[[_{1}^{2}H]\]and helium \[[_{2}^{4}He]\]are 1.1 MeV and 7 MeV. The energy released when two deuterons fuse to form a helium nucleus is :
A)
32.4 MeV
done
clear
B)
23.6 MeV
done
clear
C)
16.2 MeV
done
clear
D)
11.8 MeV
done
clear
View Answer play_arrow
question_answer 60) The activity of 1 mg sample of \[_{38}^{90}sr\] whose half-life is 28 yr is : (Given that Avogadros number is \[6.02\times {{10}^{23}}\])
A)
\[5.24\times {{10}^{9}}\] disintegrations/s
done
clear
B)
\[5.24\times {{10}^{10}}\] disintegrations/s
done
clear
C)
\[5.24\times {{10}^{8}}\] disintegrations/s
done
clear
D)
\[5.24\times {{10}^{11}}\] disintegrations/s
done
clear
View Answer play_arrow
question_answer 61) Atomic radius of fee is :
A)
\[\frac{a}{2}\]
done
clear
B)
\[\frac{a}{2\sqrt{2}}\]
done
clear
C)
\[\frac{\sqrt{3}}{4}a\]
done
clear
D)
\[\frac{\sqrt{3}}{2}a\]
done
clear
View Answer play_arrow
question_answer 62) n-type semiconductor is formed :
A)
when a germanium crystal is doped with a impurity containing 3 valence electrons
done
clear
B)
from pure germanium
done
clear
C)
from pure silicon
done
clear
D)
when a germanium crystal are doped with an impurity containing 5-valence of electrons
done
clear
View Answer play_arrow
question_answer 63) A solid that transmits light in visible region and has a very low melting point possesses :
A)
metallic bonding
done
clear
B)
ionic bonding
done
clear
C)
covalent bonding
done
clear
D)
van der Waals bonding
done
clear
View Answer play_arrow
question_answer 64) To use a transistor as an amplifier :
A)
both junctions are forward biased
done
clear
B)
both junctions are reverse biased
done
clear
C)
the emitter-base junction is forward biased and the collector-base junction is reverse biased
done
clear
D)
no biasing voltages are required
done
clear
View Answer play_arrow
question_answer 65) Huygens principle cannot explain :
A)
origin of spectra
done
clear
B)
refraction
done
clear
C)
reflection
done
clear
D)
diffraction
done
clear
View Answer play_arrow
question_answer 66) Which of the following phenomenon is not shown by sound waves?
A)
Diffraction
done
clear
B)
Interference
done
clear
C)
Polarisation
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 67) A thin equilateral triangle has a refractive index of \[\sqrt{3}\]. Find the angle of minimum deviation :
A)
\[30{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[90{}^\circ \]
done
clear
View Answer play_arrow
question_answer 68) Which of the following does not change when a wave travels from one medium to another medium?
A)
Wavelength
done
clear
B)
Frequency
done
clear
C)
Velocity
done
clear
D)
Amplitude
done
clear
View Answer play_arrow
question_answer 69) The critical angle of a prism is 30°. The velocity of light in the medium is :
A)
\[1.5\times {{10}^{8}}m/s\]
done
clear
B)
\[3\times {{10}^{8}}m/s\]
done
clear
C)
\[4.5\times {{10}^{8}}m/s\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 70) In order to see diffraction the thickness of the film is:
A)
100 \[\overset{0}{\mathop{A}}\,\]
done
clear
B)
10,000 \[\overset{0}{\mathop{A}}\,\]
done
clear
C)
1 mm
done
clear
D)
1 cm
done
clear
View Answer play_arrow
question_answer 71) An equation \[x={{t}^{3}}-2f\] denotes relationship between displacement and time. At t = 4 s, acceleration is given by:
A)
12 unit
done
clear
B)
22 unit
done
clear
C)
24 unit
done
clear
D)
26 unit
done
clear
View Answer play_arrow
question_answer 72) Light is an electromagnetic wave. Its speed in vacuum is given by the expression :
A)
\[\sqrt{{{\mu }_{0}}{{\varepsilon }_{0}}}\]
done
clear
B)
\[\sqrt{\frac{{{\mu }_{0}}}{{{\varepsilon }_{0}}}}\]
done
clear
C)
\[\sqrt{\frac{{{\varepsilon }_{0}}}{{{\mu }_{0}}}}\]
done
clear
D)
\[\frac{1}{\sqrt{{{\mu }_{0}}{{\varepsilon }_{0}}}}\]
done
clear
View Answer play_arrow
question_answer 73) In Youngs experiment, the ratio of maximum and minimum intensities in the fringe system is 9:1. The ratio of amplitudes of coherent sources is :
A)
9 : 1
done
clear
B)
3:1
done
clear
C)
2 : 1
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 74) A nucleus represented by the symbol ZXA has:
A)
Z neutrons and (A - Z) protons
done
clear
B)
Z protons and (A - Z) neutrons
done
clear
C)
Z protons and A neutrons
done
clear
D)
A protons and (Z - A) neutrons
done
clear
View Answer play_arrow
question_answer 75) A radioactive substance decays to \[\frac{1}{16}\] th of its initial activity in 40 days. The half-life of the radioactive substance expressed in days is:
A)
2.5
done
clear
B)
5
done
clear
C)
10
done
clear
D)
20
done
clear
View Answer play_arrow
question_answer 76) How many atoms of sulphur are present in 0.2 mole of sulphur\[({{S}_{8}})\]molecule?
A)
\[9.64\times {{10}^{23}}\]
done
clear
B)
\[96.4\times {{10}^{23}}\]
done
clear
C)
\[1.205\times {{10}^{23}}\]
done
clear
D)
\[12.05\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 77) The change\[_{14}^{30}Si\xrightarrow[{}]{{}}_{15}^{30}P\]requires the emission of:
A)
\[\alpha -\]particle
done
clear
B)
\[\beta -\]particle
done
clear
C)
neutron
done
clear
D)
positron
done
clear
View Answer play_arrow
question_answer 78) \[_{19}^{39}K\]and\[_{20}^{40}Ca\]are:
A)
isomers
done
clear
B)
isobars
done
clear
C)
isotones
done
clear
D)
isotopes
done
clear
View Answer play_arrow
question_answer 79) The pH of\[0.1\text{ }N\text{ }NaOH\]solution will be:
A)
0
done
clear
B)
1
done
clear
C)
7
done
clear
D)
13
done
clear
View Answer play_arrow
question_answer 80) Which of the following can behave as amphoteric oxide?
A)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
B)
\[N{{a}_{2}}O\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 81) Which of the following value of\[{{n}_{1}}\] in the relationship\[\frac{1}{\lambda }=R\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\] is correct when\[{{n}_{2}}>{{n}_{1}}\]corresponds to Paschen lines in the hydrogen spectrum?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 82) The correct set of quantum numbers for unpaired electron of fluorine is:
A)
\[n=3,l=1,\,m=0\]
done
clear
B)
\[n=2,l=1,\text{ }m=0\]
done
clear
C)
\[n=2,l=0,m=0\]
done
clear
D)
\[n=2,l=1,\text{ }m=1\]
done
clear
View Answer play_arrow
question_answer 83) Calculate the amount of\[[Ca{{(OH)}_{2}}]\]required to remove the hardness of water from 60,000 L containing 16.2 g of\[Ca{{(HC{{O}_{3}})}_{2}}\]per 100 L:
A)
1.11 kg
done
clear
B)
2.22kg
done
clear
C)
3.33 kg
done
clear
D)
4.44 kg
done
clear
View Answer play_arrow
question_answer 84) Precipitation takes place when then product of concentration of ions is:
A)
more than solubility product
done
clear
B)
negligible
done
clear
C)
less than the solubility product
done
clear
D)
equals the solubility product
done
clear
View Answer play_arrow
question_answer 85) Which of the following is an emulsion?
A)
Milk
done
clear
B)
White of an egg
done
clear
C)
Cheese
done
clear
D)
Soap solution
done
clear
View Answer play_arrow
question_answer 86) The presence of colloidal particles can be confirmed with the help of:
A)
naked eye
done
clear
B)
ordinary microscope
done
clear
C)
ultra-microscope
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 87) Which of the following is an example of homogeneous catalysis?
A)
\[2S{{O}_{2}}+{{O}_{2}}\xrightarrow{NO}2S{{O}_{3}}\]
done
clear
B)
\[2CO(g)+2{{H}_{2}}(g)\xrightarrow{ZnO}C{{H}_{3}}OH\]
done
clear
C)
\[2S{{O}_{2}}+{{O}_{2}}\xrightarrow{{{V}_{2}}{{O}_{5}}}2S{{O}_{3}}\]
done
clear
D)
\[2CO(g)+{{O}_{2}}(g)\xrightarrow{{{V}_{2}}{{O}_{5}}}2C{{O}_{2}}(g)\]
done
clear
View Answer play_arrow
question_answer 88) An aqueous solution contains 25% ethanol and 50% acetic acid by mass. Calculate the mole fraction of acetic acid in this solution:
A)
0.196
done
clear
B)
0.301
done
clear
C)
0.392
done
clear
D)
0.503
done
clear
View Answer play_arrow
question_answer 89) If a given sample of water contains 0.06 g of\[C{{a}^{2+}}\]ion per kg, calculate the ppm level of\[C{{a}^{2+}}\]ions:
A)
0.6
done
clear
B)
60
done
clear
C)
6
done
clear
D)
600
done
clear
View Answer play_arrow
question_answer 90) Which of the following solution shows positive deviation?
A)
Water + nitric acid
done
clear
B)
Acetone + aniline
done
clear
C)
Acetone + benzene
done
clear
D)
Acetone + chloroform
done
clear
View Answer play_arrow
question_answer 91) Calculate the osmotic pressure of a solution containing 0.153 g glucose in 0.1 L of the solution at 298 K?
A)
0.027 atm
done
clear
B)
0.107 atm
done
clear
C)
0.172 atm
done
clear
D)
0.207 atm
done
clear
View Answer play_arrow
question_answer 92) If the enthalpy change for the reaction is\[2N{{H}_{3}}(g)\xrightarrow{{}}{{N}_{2}}(g)+3{{H}_{2}}(g)\]is\[92\,kJ\,mo{{l}^{-1}}\] the enthalpy of formation of ammonia is:
A)
\[-23.0\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
B)
\[-46\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
C)
\[-92.0\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
D)
\[46\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 93) The heat of solution depends upon:
A)
nature of solute
done
clear
B)
nature of solvent
done
clear
C)
concentration of the solution
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 94) The enthalpy of neutralization of\[NaOH\]with acetic acid is 55 kJ and with\[HCl\]is\[57.1\text{ }kJ\]. This happens because:
A)
some heat is required for complete ionisation of acetic acid
done
clear
B)
acetic acid is an organic acid
done
clear
C)
acetic acid is less soluble in water
done
clear
D)
acetic acid is a weak acid and required lesser alkali
done
clear
View Answer play_arrow
question_answer 95) \[In\text{ }the\text{ }reaction\text{ }milk\to cheese,\text{ }\Delta \text{S}\]is:
A)
0
done
clear
B)
negative
done
clear
C)
positive
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 96) How many Faradays of electricity are required. to be passed through an aqueous solution of\[AlC{{l}_{3}}\]for deposition of 13.5 g of aluminium metal?
A)
0.5
done
clear
B)
1.0
done
clear
C)
1.5
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 97) Which substance is acting as a reducing agent in the reaction? \[2MnO_{4}^{-}+16{{H}^{+}}+10F{{e}^{2+}}\xrightarrow{{}}2M{{n}^{2+}}\]\[+10F{{e}^{3+}}+8{{H}_{2}}O\]
A)
\[{{H}_{2}}O\]
done
clear
B)
\[{{H}^{+}}\]
done
clear
C)
\[F{{e}^{2+}}\]
done
clear
D)
\[MnO_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 98) The space in the dry cell is filled with:
A)
paste of\[KOH\]and\[ZnO\]
done
clear
B)
\[Mn{{O}_{2}},ZnC{{l}_{2}},\]a filter
done
clear
C)
\[Mn{{O}_{2}},ZnC{{l}_{2}},N{{H}_{4}}Cl\] and a filter
done
clear
D)
\[MnC{{l}_{2}},ZnC{{l}_{2}},N{{H}_{4}}Cl\]and a filter
done
clear
View Answer play_arrow
question_answer 99) Measurement of the dry gas from the volume of moist gas is based on:
A)
Boyles law
done
clear
B)
Avogadros law
done
clear
C)
Gay Lussacs law
done
clear
D)
Daltons law of partial pressure
done
clear
View Answer play_arrow
question_answer 100) According to kinetic theory of gases
A)
the absolute temperature is a measure of kinetic energy of molecules
done
clear
B)
molecules attract each other
done
clear
C)
energy is lost during molecular collision
done
clear
D)
molecules possess appreciable volume
done
clear
View Answer play_arrow
question_answer 101) To which of the crystal systems does\[Ti{{O}_{2}}\] (titanium oxide) belongs?
A)
Tetragonal
done
clear
B)
Cubic
done
clear
C)
Hexagonal
done
clear
D)
Monoclinic
done
clear
View Answer play_arrow
question_answer 102) Which of the following non-metal possesses the atomicity double than that of phosphorus?
A)
Oxygen
done
clear
B)
Sulphur
done
clear
C)
Nitrogen
done
clear
D)
Arsenic
done
clear
View Answer play_arrow
question_answer 103) Which of the following has least ionic radius?
A)
\[{{I}^{+}}\]
done
clear
B)
\[{{I}^{3+}}\]
done
clear
C)
\[{{I}^{5+}}\]
done
clear
D)
\[{{I}^{7+}}\]
done
clear
View Answer play_arrow
question_answer 104) Which of the following has highest ionization energy?
A)
As
done
clear
B)
F
done
clear
C)
Ne
done
clear
D)
He
done
clear
View Answer play_arrow
question_answer 105) Which of the following has highest electron affinity?
A)
Argon
done
clear
B)
Chlorine
done
clear
C)
Fluorine
done
clear
D)
Oxygen
done
clear
View Answer play_arrow
question_answer 106) Which of the following has the same number of unpaired electrons as are present in samarium?
A)
Scandium
done
clear
B)
Chromium
done
clear
C)
Manganese
done
clear
D)
Iron
done
clear
View Answer play_arrow
question_answer 107) In which of the following compounds manganese has oxidation number equal to that of sulphur in manganese (II) sulphate?
A)
potassium permanganate
done
clear
B)
manganous chloride
done
clear
C)
potassium manganite
done
clear
D)
manganese dioxide
done
clear
View Answer play_arrow
question_answer 108) Which of the following has no unpaired electrons but is coloured?
A)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]
done
clear
B)
\[{{K}_{2}}Mn{{O}_{4}}\]
done
clear
C)
\[CuS{{O}_{4}}.5{{H}_{2}}O\]
done
clear
D)
\[MnC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 109) Which of the following is expected not to yield a white precipitate with silver nitrate?
A)
\[[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]\]
done
clear
B)
\[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl\]
done
clear
C)
\[[Co{{(N{{H}_{3}})}_{5}}Cl]C{{l}_{2}}\]
done
clear
D)
\[[Co{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 110) Which of the following ligand can yield linkage isomers?
A)
\[CO_{3}^{2-}\]
done
clear
B)
\[N{{O}_{3}}\]
done
clear
C)
\[NO_{2}^{-}\]
done
clear
D)
\[CIO_{4}^{-}\]
done
clear
View Answer play_arrow
question_answer 111) Which of the -following complexes is tetrahedral and do not have any unpaired electrons?
A)
\[Ni{{[{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
B)
\[{{K}_{2}}[NiC{{l}_{4}}]\]
done
clear
C)
\[{{K}_{2}}[Ni{{(CN)}_{4}}]\]
done
clear
D)
\[[Ni{{(CO)}_{4}}]\]
done
clear
View Answer play_arrow
question_answer 112) Doubling the concentration of A in the reaction\[A\to B\]increases the rate of formation of B by a factor of 4. The order of reaction kinetics is:
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 113) Rate constants of some of the reactions are given below. Which one belongs to third order reaction?
A)
\[8.7\times {{10}^{-3}}L\text{ }mo{{l}^{-1}}{{s}^{-1}}\]
done
clear
B)
6.5 mol
done
clear
C)
\[5.3\times {{10}^{-5}}{{s}^{-1}}\]
done
clear
D)
\[2.5\times {{10}^{-2}}{{L}^{2}}mo{{l}^{-2}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 114) The half-life period of a reaction is independent of initial pressure. Predict the order of the reaction.
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 115) Which of the following has a linear shape?
A)
\[{{H}_{2}}O\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[B{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 116) Which of the following is the weakest bond?
A)
Hydrogen bond
done
clear
B)
Metallic bond
done
clear
C)
Ionic bond
done
clear
D)
Covalent bond
done
clear
View Answer play_arrow
question_answer 117) Which of the following is a\[f-\]block element?
A)
Ce
done
clear
B)
Cs
done
clear
C)
Cu
done
clear
D)
Cr
done
clear
View Answer play_arrow
question_answer 118) The total number of monobromo derivatives of isohexane are:
A)
6
done
clear
B)
5
done
clear
C)
4
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 119) The specific rotation of (R) (-)\[2-\]bromooctane is\[-\,40\]. What is the percentage composition of mixture of enantiomers of\[2-\]bromooctane whose rotation is\[+20\]?
A)
The mixture has 25% R and 75% S
done
clear
B)
(b )The mixture has 20% R and 80% S
done
clear
C)
The mixture has 50% R and 50% S
done
clear
D)
The mixture has 75% R and 25% S
done
clear
View Answer play_arrow
question_answer 120) Cracking process is specifically directed towards one of the following:
A)
refining of petroleum
done
clear
B)
production of gas oil
done
clear
C)
production of complex chemicals
done
clear
D)
production of fuel
done
clear
View Answer play_arrow
question_answer 121) If ozonolysis of an organic compound A yields acetone and propionaldehyde, then A is:
A)
iso-butylene
done
clear
B)
3-methyl-2-pentene
done
clear
C)
2-methyl-2-pentene
done
clear
D)
2-methyl-1-peniene
done
clear
View Answer play_arrow
question_answer 122) For the industrial manufacture of high density polyethylene, identify the process that is generally adopted out of following:
A)
free-radical liquid phase of polymerization
done
clear
B)
free-radical gas phase polymerization
done
clear
C)
Zeigler-Natta or oxide catalyzed polymerization
done
clear
D)
anionic polymerization
done
clear
View Answer play_arrow
question_answer 123) In the manufacture of nylon-66, balance of functional group is ensured by adopting one of the following:
A)
preparing and purifying the nylon salt in a separate stage
done
clear
B)
by using excess of adipic acid or hexamethylene diamine
done
clear
C)
by using excess of pressure in the process
done
clear
D)
by the choice of appropriate catalysts
done
clear
View Answer play_arrow
question_answer 124) Which of the following is as per the definition of electron-affinity?
A)
\[{{X}_{2}}(g)\xrightarrow{+2{{e}^{-}}}2{{X}^{-}}(g)\]
done
clear
B)
\[X(g)\xrightarrow{+{{e}^{-}}}{{X}^{-}}(g)\]
done
clear
C)
\[{{X}^{+}}(g)\xrightarrow{+{{e}^{-}}}X(g)\]
done
clear
D)
\[{{X}^{-}}(g)\xrightarrow{+{{e}^{-}}}{{X}^{2-}}(g)\]
done
clear
View Answer play_arrow
question_answer 125) Which of the following is a soda ash?
A)
\[N{{a}_{2}}C{{O}_{3}}-10{{H}_{2}}O\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}-7{{H}_{2}}O\]
done
clear
C)
\[N{{a}_{2}}C{{O}_{3}}-6{{H}_{2}}O\]
done
clear
D)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 126) Coke is used in metallurgical process as:
A)
slag
done
clear
B)
flux
done
clear
C)
reducing agent
done
clear
D)
oxidising agent
done
clear
View Answer play_arrow
question_answer 127) Which of the following is iron-pyrites?
A)
\[F{{e}_{2}}{{O}_{3}}-3{{H}_{2}}O\]
done
clear
B)
\[FeS\]
done
clear
C)
\[Fe{{S}_{2}}\]
done
clear
D)
\[F{{e}_{2}}{{S}_{3}}\]
done
clear
View Answer play_arrow
question_answer 128) Which of the following is malachite?
A)
\[CuC{{O}_{3}}.Cu{{(OH)}_{2}}\]
done
clear
B)
\[2CuC{{O}_{3}}.Cu{{(OH)}_{2}}\]
done
clear
C)
\[C{{u}_{2}}O\]
done
clear
D)
\[C{{u}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 129) How many structural isomers can be written for an organic compound with molecular formula\[{{C}_{5}}{{H}_{11}}Br\]?
A)
5
done
clear
B)
7
done
clear
C)
4
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 130) \[RCl\]is treated with Li in ether solution to form\[RLi.\text{ }RLi\]reacts with water to form is opentane.\[RCl\] also reacts with Na to form 2, 7-dimethyl-octane. What is the structure of\[RCl\]?
A)
Octyl-chloride
done
clear
B)
2-chloro-2-methyl-butane
done
clear
C)
Dimethyl-chloro-octane
done
clear
D)
Isopentyl-chloride
done
clear
View Answer play_arrow
question_answer 131) What is the oxidation number of C in\[C{{H}_{3}}Cl\]?
A)
\[-\,3\]
done
clear
B)
0
done
clear
C)
\[-\,2\]
done
clear
D)
\[-\,4\]
done
clear
View Answer play_arrow
question_answer 132) The product of glycine with aqueous\[HCl\]is:
A)
\[{{H}_{2}}N-CHCl-COOH\]
done
clear
B)
\[\overline{C}l{{H}_{3}}\overset{\oplus }{\mathop{N}}\,-C{{H}_{2}}-COOH\]
done
clear
C)
\[{{H}_{2}}N-C{{H}_{2}}-COCl\]
done
clear
D)
\[{{H}_{2}}N-CHCl-CO\overline{O}\overset{\oplus }{\mathop{C}}\,l\]
done
clear
View Answer play_arrow
question_answer 133) When RNA is hydro lysed the quantities of the four bases obtained are generally unequal, unlike DNA. What does this fact suggest about the structure of RNA?
A)
A double strand helix
done
clear
B)
A single strand helix
done
clear
C)
An equal mixture of double and single strand helix
done
clear
D)
Unequal mixture of double and single strand
done
clear
View Answer play_arrow
question_answer 134) The prosthetic group of protein rhodopsin is :
A)
\[\beta -\]carotene
done
clear
B)
vitamin-A
done
clear
C)
11-cis-retinol
done
clear
D)
retinol
done
clear
View Answer play_arrow
question_answer 135) The diazodzation of two feebly basic aromatic amines is achieved by using:
A)
nitrous acid
done
clear
B)
nitrosyl-hydrochloric acid
done
clear
C)
sulphuric acid
done
clear
D)
nitrosoyl-sulphuric acid
done
clear
View Answer play_arrow
question_answer 136) What kind of amines can be tested by reaction with chloroform under basic conditions?
A)
Primary aliphatic amines
done
clear
B)
Primary aromatic amines
done
clear
C)
Both primary aliphatic and aromatic amines
done
clear
D)
All kind of available amines
done
clear
View Answer play_arrow
question_answer 137) Which reagent can be used for the alkylation of aromatic nitro compounds?
A)
Methyl-sulfinyl carbanion
done
clear
B)
Methyl-iodide
done
clear
C)
Dimethyl-sulphate
done
clear
D)
Methyl-lithium
done
clear
View Answer play_arrow
question_answer 138) What is the product of reduction of a carboxylic ester using a reagent prepared from\[B{{F}_{3}}-\]etherate and\[LiAl{{H}_{4}}\]?
A)
A carboxyiic acid
done
clear
B)
An alcohol
done
clear
C)
A cyclic ester
done
clear
D)
An ether
done
clear
View Answer play_arrow
question_answer 139) The formylation of certain aromatic compounds using carbon monoxide and\[HCl\] in presence of cuprous chloride is known as Gattermann-Koch reaction and the product of this reaction is represented as:
A)
\[ArCOCl\]
done
clear
B)
\[ArH\]
done
clear
C)
\[ArCHO\]
done
clear
D)
\[ArCuCl\]
done
clear
View Answer play_arrow
question_answer 140) Gem-dihalides are hydrolysed with either acid or basic catalysts to give:
A)
aldehydes or ketones
done
clear
B)
halohydrins
done
clear
C)
carboxylic acids
done
clear
D)
gem-diols
done
clear
View Answer play_arrow
question_answer 141) What is A in the following reaction? \[{{H}_{3}}C-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-R\xrightarrow[O{{H}^{-}}]{B{{r}_{2}}}CHB{{r}_{3}}+A\]
A)
\[R-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-R\]
done
clear
B)
\[R-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-{{O}^{-}}\]
done
clear
C)
\[R-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-Br\]
done
clear
D)
\[R-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-OR\]
done
clear
View Answer play_arrow
question_answer 142) The difference in activity: \[M{{e}_{2}}CC{{H}_{2}}COOH>M{{e}_{3}}SiC{{H}_{2}}COOH\]can be attributed to the fact diat:
A)
silicon is more electropositive than carbon and therefore has an electron donating inductive effect
done
clear
B)
silicon is a non-metal
done
clear
C)
carbon is more electropositive than silicon and has an electron donating inductive effect
done
clear
D)
carbon is a non-metal
done
clear
View Answer play_arrow
question_answer 143) 3-pentanol upon reaction with\[PB{{r}_{3}}\] gives 2 and 3-bromopentane. Such rearrangement can be avoided by:
A)
using excess of\[PB{{r}_{3}}\]
done
clear
B)
choosing low temperature for reaction
done
clear
C)
converting alcohol into sulphuric or sulphonic acid
done
clear
D)
converting alcohol into carboxylate esters
done
clear
View Answer play_arrow
question_answer 144) The considerably greater acid strength of \[PhOH(p{{K}_{a}}=10)\]than that of \[ROH(p{{K}_{a}}=18)\]is due to the fact that:
A)
\[PhO\](phenoxide ion) is a stronger base than\[R{{O}^{-}}\](alkoxide ion)
done
clear
B)
\[PhO\](phenoxide ion) is a weaker base than \[R{{O}^{-}}\](alkoxide ion)
done
clear
C)
\[ROH\]is soluble in water
done
clear
D)
\[PhOH\]is aromatic in nature
done
clear
View Answer play_arrow
question_answer 145) The correct order of relative acidic strength of the following compounds is:
A)
phenol > o-nitrophenol > m-nitrophenol > p-nitrophenol
done
clear
B)
p-nitrophenol > m-nitrophenol > o-nitrophenol > phenol
done
clear
C)
p-nitrophenol > o-nitrophenol > m-nitrophenol > phenol
done
clear
D)
o-nitrophenol > m-nitrophenol > p-nitrophenol > phenol
done
clear
View Answer play_arrow
question_answer 146) A radioactive isotope has a half-life of 10 days. What was its original weight 40 days earlier if today it is 125 g?
A)
600 g
done
clear
B)
1000 g
done
clear
C)
1250 g
done
clear
D)
2000 g
done
clear
View Answer play_arrow
question_answer 147) For the reversible system: \[X(g)Y(g)+Z(g),\]a quantity of X was heated at constant pressure P at a certain temperature. The equilibrium partial pressure of X was found to be\[\frac{P}{7}\]. What is the value of \[{{K}_{p}}\]at given temperature?
A)
\[\frac{6P}{7}\]
done
clear
B)
\[\frac{9P}{7}\]
done
clear
C)
\[\frac{36P}{7}\]
done
clear
D)
\[6P\]
done
clear
View Answer play_arrow
question_answer 148) The equilibrium constant for a reversible chemical reaction varies with T as: \[\begin{matrix} {{K}_{p}}(at{{m}^{-2}}) & {{10}^{-2}} & {{10}^{-3}} \\ T(K) & 400 & 450 \\ \end{matrix}\] From this, it may be deduced that:
A)
the value of\[{{K}_{p}}\]increases with increase in temperature
done
clear
B)
the forward reaction gives out heat
done
clear
C)
there are more moelcules on the right hand side of the chemical equation than on the left
done
clear
D)
the reaction proceeds ten times faster at 450 K than 400 K
done
clear
View Answer play_arrow
question_answer 149) Slaked lime,\[Ca{{(OH)}_{2}}\]is used extensively in sewage treatment. What is the maximum pH that can be established in\[Ca{{(OH)}_{2}}(aq)\]? \[Ca{{(OH)}_{2}}(g)C{{a}^{2+}}(aq.)+2O{{H}^{-}}(aq);\]\[{{K}_{sp}}=5.5\times {{10}^{-6}}\]
A)
1.66
done
clear
B)
12.3424
done
clear
C)
7.0
done
clear
D)
14.0
done
clear
View Answer play_arrow
question_answer 150) Calculate the first dissociation constant of\[{{H}_{3}}P{{O}_{4}}\]if the emf of the cell: \[Hg|H{{g}_{2}}C{{l}_{2}}(s)|KCl(salt)||{{H}_{3}}P{{O}_{4}}(0.1)M;\]\[Na{{H}_{2}}P{{O}_{4}}(0.1)|{{H}_{2}}(1\,atm)pt\] is \[-0.3665\text{ }V.\text{ E}_{red}^{o}\,of\text{ }SHE=0.2412\text{ }V\]
A)
\[7.583\times {{10}^{-5}}\]
done
clear
B)
\[6.583\times {{10}^{-5}}\]
done
clear
C)
\[7.03\times {{10}^{-5}}\]
done
clear
D)
\[7.583\times {{10}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 151) All cells contain:
A)
mitochondria and nucleus
done
clear
B)
Golgi bodies
done
clear
C)
chloroplast
done
clear
D)
ribosomes
done
clear
View Answer play_arrow
question_answer 152) In cells, centrioles occur:
A)
singly
done
clear
B)
in duplets
done
clear
C)
in triplets
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 153) Golgi bodies are absent in :
A)
RBC
done
clear
B)
sieve tubes
done
clear
C)
prokaryotes
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 154) Plasma membrane is made up of:
A)
carbohydrates and fats
done
clear
B)
carbohydrates and proteins
done
clear
C)
proteins and fats
done
clear
D)
fats only
done
clear
View Answer play_arrow
question_answer 155) Which of the following gives mechanical support to the cell?
A)
Chloroplasts
done
clear
B)
Mitochondria
done
clear
C)
Ribosomes
done
clear
D)
Endoplasmic reticulum
done
clear
View Answer play_arrow
question_answer 156) Which of the following constitutes unit membrane?
A)
Golgian membrane
done
clear
B)
ER membrane
done
clear
C)
Plasma membrane
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 157) In ribosomes letter S signifies :
A)
sedimentation coefficient
done
clear
B)
Svedbergs constant
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 158) Showy and attractive colour of fruits is due to the presence of:
A)
chloroplasts
done
clear
B)
leucoplasts
done
clear
C)
chromoplasts
done
clear
D)
amvloplasts
done
clear
View Answer play_arrow
question_answer 159) Cell was discovered by :
A)
Robert Brown
done
clear
B)
Robert Hooke
done
clear
C)
Camillo Golgi
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 160) Mitochondria are absent in :
A)
red algae
done
clear
B)
green algae
done
clear
C)
brown algae
done
clear
D)
bacteria
done
clear
View Answer play_arrow
question_answer 161) Cell theory was proposed by :
A)
Robert Hooke
done
clear
B)
Schleiden and Schwann
done
clear
C)
Robert Brown
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 162) Predominant pith is found in:
A)
dicot root and monocot stem
done
clear
B)
dicot stem and dicot root
done
clear
C)
dicot stem and monocot stem
done
clear
D)
dicot stem and monocot root
done
clear
View Answer play_arrow
question_answer 163) Dorsiventral leaf has:
A)
spongy parenchyma on upper side
done
clear
B)
spongy parenchyma on both side
done
clear
C)
palisade parenchyma on lower side
done
clear
D)
palisade parenchyma on upper side
done
clear
View Answer play_arrow
question_answer 164) Monocots do not show secondary growth because their vascular bundles are:
A)
open
done
clear
B)
closed
done
clear
C)
scattered
done
clear
D)
radial
done
clear
View Answer play_arrow
question_answer 165) Vascular cambium is a :
A)
primary meristem
done
clear
B)
intercalary meristem
done
clear
C)
secondary growth
done
clear
D)
lateral meristem
done
clear
View Answer play_arrow
question_answer 166) The net gain of ATP in anaerobic respiration is:
A)
2
done
clear
B)
4
done
clear
C)
36
done
clear
D)
32
done
clear
View Answer play_arrow
question_answer 167) Citric acid cycle is also called :
A)
Krebs cycle
done
clear
B)
TCA cycle
done
clear
C)
tricarboxylic acid cycle
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 168) Which of the following fungus is related to discovery of gibberellins ?
A)
Fusarium pernoesum
done
clear
B)
Fusarium noriform
done
clear
C)
Fusarium monoliformae
done
clear
D)
ergot fungus
done
clear
View Answer play_arrow
question_answer 169) Which of the following is not an auxin?
A)
IAA
done
clear
B)
IBA
done
clear
C)
NAA
done
clear
D)
Zeatin
done
clear
View Answer play_arrow
question_answer 170) A fixed board is at a height of 2 m from the ground level on the tree which grows 1.5 m per year. What is the height of the board after 5 years ?
A)
2 m
done
clear
B)
7.5 m
done
clear
C)
5 m
done
clear
D)
7 m
done
clear
View Answer play_arrow
question_answer 171) Chlamydornonas reproduces by :
A)
zoospore formation
done
clear
B)
aplanospore formation
done
clear
C)
hypnospore formation
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 172) In Rhizopus heterothalism occurs which signifies that:
A)
torulla stage is found in Rhizopus
done
clear
B)
it reproduces parthenogenetically
done
clear
C)
two compatible opposite strains come close and their swollen ends join to form zygospore
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 173) In Pinus :
A)
seed is winged but pollen grains are not
done
clear
B)
both seed and pollen grains are wingless
done
clear
C)
pollen grains are winged but seed is not
done
clear
D)
both seeds and pollen grains are winged
done
clear
View Answer play_arrow
question_answer 174) Study of flowers is called :
A)
Anthology
done
clear
B)
Pomology
done
clear
C)
Floriculture
done
clear
D)
Spermolgy
done
clear
View Answer play_arrow
question_answer 175) Viability of seeds can be tested by :
A)
triphenyl chloride
done
clear
B)
IAA
done
clear
C)
mercuric chloride
done
clear
D)
2, 4-D
done
clear
View Answer play_arrow
question_answer 176) The first product of \[C{{O}_{2}}\] fixation in \[{{C}_{4}}\]plants is:
A)
PGA
done
clear
B)
oxaloacetic acid
done
clear
C)
malic acid
done
clear
D)
PEP
done
clear
View Answer play_arrow
question_answer 177) Pollination by bats is called :
A)
ornithophily
done
clear
B)
anemophily
done
clear
C)
entomophily
done
clear
D)
chiropterophily
done
clear
View Answer play_arrow
question_answer 178) Meiotic divisions are required for forming 100 zygotes/grain of wheat is :
A)
100
done
clear
B)
75
done
clear
C)
125
done
clear
D)
50
done
clear
View Answer play_arrow
question_answer 179) Seed is:
A)
developed ovule
done
clear
B)
developed ova 17
done
clear
C)
fertilized and developed ovule
done
clear
D)
fertilized and developed ovary
done
clear
View Answer play_arrow
question_answer 180) Selection of homozygous plant is :
A)
mass selection
done
clear
B)
pure line selection
done
clear
C)
mixed selection
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 181) Emasculation means :
A)
removal of carpels
done
clear
B)
removal of anthers
done
clear
C)
removal of pistils
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 182) Which of the following is best fertilizer for paddy ?
A)
Azolla pinnaca
done
clear
B)
Bacillus polymixa
done
clear
C)
Anthoceros
done
clear
D)
Rhizobium
done
clear
View Answer play_arrow
question_answer 183) DEVINER and COLLEGOR are two trade names in agriculture. They are used as :
A)
bioinsecticides
done
clear
B)
natural insecticides
done
clear
C)
biofungicides
done
clear
D)
bioherbicides
done
clear
View Answer play_arrow
question_answer 184) The process by which language of DNA is covnerted into language of m-RNA is called :
A)
transformation
done
clear
B)
transcription
done
clear
C)
translation
done
clear
D)
transduction
done
clear
View Answer play_arrow
question_answer 185) Which of the following is a sex linked disease ?
A)
Colour blindness
done
clear
B)
Rickets
done
clear
C)
Diphtheria
done
clear
D)
Beri-beri
done
clear
View Answer play_arrow
question_answer 186) In sickle cell anaemia there is deformity of:
A)
WBCs
done
clear
B)
platelets
done
clear
C)
RBCs
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 187) Number of autosomes in human sex cells is :
A)
22
done
clear
B)
12
done
clear
C)
44
done
clear
D)
23
done
clear
View Answer play_arrow
question_answer 188) Which of the following is not seen in RNA?
A)
Thymine
done
clear
B)
Uracil
done
clear
C)
Adenine
done
clear
D)
Guanine
done
clear
View Answer play_arrow
question_answer 189) Meiosis occurs in :
A)
somatic cells only
done
clear
B)
germ cells only
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 190) Science dealing with factors which improve successive generations of humans is called :
A)
Euphenics
done
clear
B)
Euthenics
done
clear
C)
Eugenics
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 191) A person met with an accident and there is no time to check his blood group, which of the following must be given to him?
A)
\[OR{{h}^{+}}\]
done
clear
B)
\[OR{{h}^{-}}\]
done
clear
C)
\[ABR{{h}^{+}}\]
done
clear
D)
\[ABR{{h}^{-}}\]
done
clear
View Answer play_arrow
question_answer 192) Nissls granules are characteristic of:
A)
muscle tissue
done
clear
B)
connective tissue
done
clear
C)
nerve tissue
done
clear
D)
epithelial tissue
done
clear
View Answer play_arrow
question_answer 193) Urea production occurs in :
A)
kidneys
done
clear
B)
ureters
done
clear
C)
urinary bladder
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 194) Villi present in intestine help in :
A)
secreting enzymes
done
clear
B)
increasing surface area for absorption
done
clear
C)
protection
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 195) Heart beat originates from
A)
AV node
done
clear
B)
SA node
done
clear
C)
AV bundle
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 196) The pigment rhodopsin is present in :
A)
choroids
done
clear
B)
retina
done
clear
C)
sclera
done
clear
D)
cornea
done
clear
View Answer play_arrow
question_answer 197) A hypermetropic person :
A)
can see far objects
done
clear
B)
can see nearer objects
done
clear
C)
cannot see in the above cases
done
clear
D)
can see in both (a) and (b)
done
clear
View Answer play_arrow
question_answer 198) The hormone which regulates reabsorption of water in kidney tubules is :
A)
ACTH from adenohypophysis
done
clear
B)
ACTH from adrenal cortex
done
clear
C)
oxytocin from neurohypophysis
done
clear
D)
ADH (vasopressin) from neurohypophysis
done
clear
View Answer play_arrow
question_answer 199) Surgical removal of vas deferens is called :
A)
tubectomy
done
clear
B)
vasectomy
done
clear
C)
vasectolysis
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 200) Fertilization of ovum in human being occurs in:
A)
fallopian tube
done
clear
B)
cervix
done
clear
C)
Fund
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 201) Release of mature Graafian follicle from ovary is called :
A)
menstruation
done
clear
B)
ovulation
done
clear
C)
estrous cycle
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 202) Islets of Langerhans occur in :
A)
liver
done
clear
B)
intestine
done
clear
C)
stomach
done
clear
D)
pancreas
done
clear
View Answer play_arrow
question_answer 203) AIDS stand for :
A)
All India Diphtherial Society
done
clear
B)
An Infants Disease Syndrome
done
clear
C)
Acquired Immune Deficiency Syndrome
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 204) Tape worm occurs as a parasite in :
A)
liver
done
clear
B)
stomach
done
clear
C)
intestine
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 205) Acid rain is caused due to :
A)
\[S{{O}_{2}}\] and \[N{{O}_{2}}\]
done
clear
B)
release of \[{{O}_{3}}\] in air
done
clear
C)
release of \[CO\] and \[C{{O}_{2}}\] in air
done
clear
D)
excessive transpiration and evaporation
done
clear
View Answer play_arrow
question_answer 206) Which of the following are ectodermal in origin?
A)
spinal cord and brain
done
clear
B)
heart and kidney
done
clear
C)
muscles
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 207) The excretory organs of platyhelminthes are :
A)
Malpighian tubules
done
clear
B)
flame cells (solenocytes)
done
clear
C)
green glands
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 208) Hydra can paralyse prey-toy :
A)
nematocysts
done
clear
B)
tentacles
done
clear
C)
mouth
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 209) Insects have :
A)
3 pairs of legs
done
clear
B)
4 pairs of legs
done
clear
C)
5 pairs of legs
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 210) Which of the following is cold blooded ?
A)
Frog
done
clear
B)
Penguin
done
clear
C)
Owl
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 211) Flight muscles of bird are attached to :
A)
keel of sternum
done
clear
B)
clavicle
done
clear
C)
pelvic girdle
done
clear
D)
humerus
done
clear
View Answer play_arrow
question_answer 212) Seismonasty represents:
A)
turgor movement
done
clear
B)
shock movement brought about by external stimuli
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 213) Chlamydornonas shows :
A)
isogamy
done
clear
B)
anisogamy
done
clear
C)
oogamy
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 214) Chilgoza pinus is :
A)
Pinus gerardiana
done
clear
B)
Pinus roxburghi
done
clear
C)
Pinus wallichiana
done
clear
D)
Pinus merkurii
done
clear
View Answer play_arrow
question_answer 215) Whales are :
A)
reptiles
done
clear
B)
mammals
done
clear
C)
fishes
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 216) Penicillin was discovered by :
A)
Robert Koch
done
clear
B)
Alexander Flemming
done
clear
C)
Louis Pasteur
done
clear
D)
Bronchi
done
clear
View Answer play_arrow
question_answer 217) Average life span of an erythrocyte is :
A)
120 days
done
clear
B)
one year
done
clear
C)
120 weeks
done
clear
D)
6 months
done
clear
View Answer play_arrow
question_answer 218) In earthworms, ovaries occur in :
A)
12th segment
done
clear
B)
27th segment
done
clear
C)
14th segment
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 219) Nucleus was discovered by :
A)
Robert Brown
done
clear
B)
Robert Hooke
done
clear
C)
Leeuwenhoek
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 220) Which of the following prevents food from going into trachea?
A)
glottis
done
clear
B)
pharynx
done
clear
C)
epiglottis
done
clear
D)
bronchi
done
clear
View Answer play_arrow
question_answer 221) Name the hormone associated with the metabolism of phosphorus and calcium?
A)
glucocorticoids
done
clear
B)
mineralocorticoids
done
clear
C)
calcitonin
done
clear
D)
glucagon
done
clear
View Answer play_arrow
question_answer 222) Which is most important in speciation?
A)
Geographical isolation
done
clear
B)
Reproductive isolation
done
clear
C)
Ecological isolation
done
clear
D)
Ethological isolation
done
clear
View Answer play_arrow
question_answer 223) Which is non-invasive technique of genetic counselling?
A)
Amniocentesis
done
clear
B)
Chorionic biopsy
done
clear
C)
Foetal blood sampling
done
clear
D)
Ultrasonography
done
clear
View Answer play_arrow
question_answer 224) Hill reaction was demonstrated :
A)
in the absence of water
done
clear
B)
in the absence of carbon dioxide
done
clear
C)
in the presence of carbon dioxide
done
clear
D)
in the absence of a suitable electron acceptor
done
clear
View Answer play_arrow
question_answer 225) In a cell cycle the RNA synthesis, needed in protein synthesis, occurs in :
A)
synthetic period
done
clear
B)
post-mitotic gap period
done
clear
C)
pre-mitotic gap period
done
clear
D)
prophase
done
clear
View Answer play_arrow