question_answer 1) Which of the following is dimensionless?
A)
Universal gravitational constant
done
clear
B)
Relative permittivity
done
clear
C)
Relative velocity
done
clear
D)
Plancks constant
done
clear
View Answer play_arrow
question_answer 2) A particle is acted upon by a force of constant magnitude which is always perpendicular to the velocity of the particle. The motion of the particle takes place in a plane. It follows :
A)
its velocity is constant
done
clear
B)
its kinetic energy is constant
done
clear
C)
its acceleration is constant
done
clear
D)
it moves in a straight line
done
clear
View Answer play_arrow
question_answer 3) Two stones are projected with the same magnitude of velocity, but making different angles with horizontal, the angle of projection of one is \[\frac{\pi }{3}\] and its maximum height is Y, the maximum height attained by the other stone with \[\frac{\pi }{6}\] angle of projection is :
A)
Y
done
clear
B)
2Y
done
clear
C)
3Y
done
clear
D)
\[\frac{\pi }{6}\]
done
clear
View Answer play_arrow
question_answer 4) A nucleus of mass number A originally at rest, emits alpha particle with speed v, the recoil speed of the daughter nucleus is:
A)
\[\frac{4v}{A-4}\]
done
clear
B)
\[\frac{4v}{A+4}\]
done
clear
C)
\[\frac{v}{A-4}\]
done
clear
D)
\[\frac{v}{A+4}\]
done
clear
View Answer play_arrow
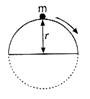
question_answer 5)
The height at which body leaves the vertical circle is:
A)
\[\frac{2}{3}r\]
done
clear
B)
\[\frac{3}{2}r\]
done
clear
C)
\[3r\]
done
clear
D)
r
done
clear
View Answer play_arrow
question_answer 6) Which of the following is a slow process?
A)
Isothermal
done
clear
B)
Adiabatic
done
clear
C)
Isobaric
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 7) A hollow cylinder and a solid cylinder of the same mass and radius are released simulta-neously from rest at the top of the same inclined plane. Which will reach the ground first?
A)
Solid cylinder
done
clear
B)
Hollow cylinder
done
clear
C)
Both will reach at the same time
done
clear
D)
Cannot be predicted
done
clear
View Answer play_arrow
question_answer 8) Let the value of acceleration due to gravity at poles and equator of earth \[{{g}_{p}}\]and \[{{g}_{e}}\]respectively. Assuming the earth to be a sphere of radius R rotating about its axis with angular speed \[\omega \] then \[{{g}_{p}}-\text{ }{{g}_{e}}\] is given by :
A)
\[\frac{{{\omega }^{2}}}{R}\]
done
clear
B)
\[R{{\omega }^{2}}\]
done
clear
C)
\[{{R}^{2}}{{\omega }^{2}}\]
done
clear
D)
\[\frac{{{\omega }^{2}}}{{{R}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 9) The orbital velocity of a satellite at a height h above the surface of earth is v. The value of escape velocity from the same location is given by:
A)
\[\sqrt{2}v\]
done
clear
B)
v
done
clear
C)
\[\frac{v}{\sqrt{2}}\]
done
clear
D)
\[\frac{v}{2}\]
done
clear
View Answer play_arrow
question_answer 10) A wire of length L, radius r, when stretched with a force F, changes in length by I What will be the change in length in a wire of same material having length 2L, radius 2r and stretched by a force 2F?
A)
\[\frac{l}{2}\]
done
clear
B)
\[l\]
done
clear
C)
\[2l\]
done
clear
D)
4L
done
clear
View Answer play_arrow
question_answer 11) A number of water droplets of radius r coalesce to form a drop of radius R. Assuming that the entire energy liberated due to coalesce goes into heating the drop, the rise in temperature d\[\theta \] is : (surface tension of water = T)
A)
\[\frac{2T}{rJ}\]
done
clear
B)
\[\frac{3T}{rJ}\]
done
clear
C)
\[\frac{3T}{J}\left[ \frac{1}{r}+\frac{1}{R} \right]\]
done
clear
D)
\[\frac{3T}{J}\left[ \frac{1}{r}-\frac{1}{R} \right]\]
done
clear
View Answer play_arrow
question_answer 12) Two equal drops of water are falling through air with a steady velocity of 5 cm/s. If the drops coalesce, the new steady velocity of the coalesced drop will be :
A)
5 cm/s
done
clear
B)
10 cm/s
done
clear
C)
7.9 cm/s
done
clear
D)
6 cm/s
done
clear
View Answer play_arrow
question_answer 13) A particle is vibrating in a simple harmonic motion with an amplitude 4 cm. At what displacement from the equilibrium is its energy half-potential and half-kinetic?
A)
\[2\sqrt{2}cm\]
done
clear
B)
\[\sqrt{2}cm\]
done
clear
C)
3 cm
done
clear
D)
1 cm
done
clear
View Answer play_arrow
question_answer 14) Liquid rises to a height of 2 cm in a capillary tube. The angle of contact between the solid and the liquid is zero. The tube is depressed more now to that the tip of the capillary tube is only 1 cm above the liquid then, the apparent angle of contact between solid and liquid is :
A)
\[0{}^\circ \]
done
clear
B)
\[30{}^\circ \]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
liquid just flows out and angle of contact is not well defined
done
clear
View Answer play_arrow
question_answer 15) Which of the following is a necessary and sufficient condition for SHM?
A)
Constant period
done
clear
B)
Constant acceleration
done
clear
C)
Proportionality between acceleration and displacement from equilibrium position
done
clear
D)
Proportionality between restoring force and displacement from equilibrium position
done
clear
View Answer play_arrow
question_answer 16) A simple pendulum suspended from the ceiling of a train has a period T when the train is at rest. When the train is accelerating with a uniform acceleration the time period of simple pendulum will:
A)
decrease
done
clear
B)
increase
done
clear
C)
remain unchanged
done
clear
D)
become infinite
done
clear
View Answer play_arrow
question_answer 17) In each fission of \[_{92}^{235}U\] energy of 200 MeV is released. How many acts of fission must occur per second to produce a power of 1 kW?
A)
\[3.1\times {{10}^{13}}\]
done
clear
B)
\[1.3\times {{10}^{16}}\]
done
clear
C)
\[1.3\times {{10}^{15}}\]
done
clear
D)
\[3.1\times {{10}^{16}}\]
done
clear
View Answer play_arrow
question_answer 18) During nuclear fusion reaction :
A)
a heavy nucleus breaks into two fragments by itself
done
clear
B)
a light nucleus bombarded by thermal neutrons breaks up
done
clear
C)
a heavy nucleus bombarded by thermal neutrons breaks up
done
clear
D)
two light nuclei combine to give a heavier nucleus and possibly other products
done
clear
View Answer play_arrow
question_answer 19) A unit cell defined by sides \[a\ne b\ne c\]and angles \[\alpha \ne \beta \ne \gamma \] is :
A)
triclinic
done
clear
B)
monoclinic
done
clear
C)
orthorhombic
done
clear
D)
hexagonal
done
clear
View Answer play_arrow
question_answer 20) n-type semiconductor is formed :
A)
when a Ge crystal is doped with an impurity containing 3 valence electrons
done
clear
B)
when a Ge crystal is doped with an impurity containing 5 valence electrons
done
clear
C)
from pure Ge crystal
done
clear
D)
from pure Si crystal
done
clear
View Answer play_arrow
question_answer 21) In a semiconductor diode, P side is earthed and N side is applied to a potential of 2 V. The diode shall :
A)
not conduct
done
clear
B)
conduct
done
clear
C)
conduct partially
done
clear
D)
breakdown
done
clear
View Answer play_arrow
question_answer 22) Which of the following is less in case of common emitter amplifier?
A)
Power gain
done
clear
B)
Current gain
done
clear
C)
Voltage gain
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 23) A well cut diamond appears bright because :
A)
it is radioactive
done
clear
B)
it emits light
done
clear
C)
it has high density
done
clear
D)
of total internal reflection
done
clear
View Answer play_arrow
question_answer 24) The angle of deviation for a prism is greater for:
A)
violet colour
done
clear
B)
blue colour
done
clear
C)
green colour
done
clear
D)
red colour
done
clear
View Answer play_arrow
question_answer 25) When light passes through a dispersive medium, it breaks up into various colours. Which of the following statements is correct?
A)
Velocity of light for violet is greater than velocity of light for red in dispersive medium
done
clear
B)
Velocity of light for violet less than the velocity of light for red in dispersive medium
done
clear
C)
Velocity of light is same for all colours in the dispersive medium
done
clear
D)
Velocity of light has nothing to do with the phenomenon of dispersion
done
clear
View Answer play_arrow
question_answer 26) A spectrum in which no frequencies between its limits are missing is called :
A)
continuous spectrum
done
clear
B)
Fraunhofer lines
done
clear
C)
emissive spectrum
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 27) According to Huygens wave theory, point on any wavefront may be regarded as :
A)
a photon
done
clear
B)
an electron
done
clear
C)
a new source of wave
done
clear
D)
neutron
done
clear
View Answer play_arrow
question_answer 28) The transverse nature of light is shown by :
A)
interference of light
done
clear
B)
diffraction
done
clear
C)
radiation spectrum of a black body
done
clear
D)
polarization
done
clear
View Answer play_arrow
question_answer 29) In a closed organ pipe, the frequency of fundamental note is 50 Hz. The note of which of the following frequencies will not be emitted by it?
A)
50 Hz
done
clear
B)
100 Hz
done
clear
C)
150 Hz
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 30) A cylindrical tube open at both ends has a fundamental frequency \[f\] in air. The tube is dipped vertically in water, so that half of it is in water. The fundamental frequency of the air column is now:
A)
\[\frac{f}{2}\]
done
clear
B)
\[\frac{3f}{4}\]
done
clear
C)
\[f\]
done
clear
D)
\[2f\]
done
clear
View Answer play_arrow
question_answer 31) The fundamental frequency of a string stretched with a weight of 4 kg is 256 Hz. The weight required to produce its octave is :
A)
4 kg-wt
done
clear
B)
8 kg-wt
done
clear
C)
12 kg-wt
done
clear
D)
16 kg-wt
done
clear
View Answer play_arrow
question_answer 32) Radiowaves of wavelength X are sent from a radar towards an aeroplane. The aeroplane is approaching towards the radar station. The wavelength of the radiowaves received after reflection from the aeroplane will be :
A)
\[\lambda \]
done
clear
B)
more than\[\lambda \]
done
clear
C)
less than \[\lambda \]
done
clear
D)
depends on speed of aeroplane
done
clear
View Answer play_arrow
question_answer 33) According to kinetic theory of gases, molecules of a gas behave like :
A)
inelastic spheres
done
clear
B)
perfectly elastic rigid spheres
done
clear
C)
perfectly elastic non-rigid spheres
done
clear
D)
inelastic non-rigid spheres
done
clear
View Answer play_arrow
question_answer 34) 70 cal of heat are required to raise the temperature of 2 moles of an ideal gas at constant pressure from \[30{}^\circ C\] to \[35{}^\circ C\]. The amount of heat required in calories to raise the temperature of the same gas through the same range \[\left( 30{}^\circ C-35{}^\circ C \right)\] at constant volume is :
A)
30 cal
done
clear
B)
50 cal
done
clear
C)
370 cal
done
clear
D)
90 cal
done
clear
View Answer play_arrow
question_answer 35) A monoatomic gas \[\left( \gamma =\frac{5}{3} \right)\]suddenly compressed to\[\frac{1}{8}\] th of the original volume adiabatically, then the pressure of the gas will become how many times larger than the pressure originally?
A)
32
done
clear
B)
\[\frac{40}{3}\]
done
clear
C)
8
done
clear
D)
same as before
done
clear
View Answer play_arrow
question_answer 36) An engineer claims to have made an engine delivering 10 kW power with fuel consumption of 1 g/s. The calorific value of the fuel is 2 kcal/g. The claim of the engineer :
A)
is valid
done
clear
B)
is invalid
done
clear
C)
depends on engine design
done
clear
D)
depends on the load
done
clear
View Answer play_arrow
question_answer 37) A sphere, a cube and a thin circular plate, all made of same material and having the same mass are initially heated to the same temperature of \[200{}^\circ C\], which of them cools fastest when left in air at room temperature:
A)
sphere
done
clear
B)
cube
done
clear
C)
circular plate
done
clear
D)
all at the same rate
done
clear
View Answer play_arrow
question_answer 38) The temperature of sun is 5500 K and it emits maximum intensity radiation in the yellow region \[\left( {{\lambda }_{m}}=5.5\times {{10}^{-7}}m \right)\] The maximum radiation from a furnace occurs at wavelength \[11\times 10\text{ }m.\]The temperature of furnace is :
A)
1125 K
done
clear
B)
2750 K
done
clear
C)
5500 K
done
clear
D)
11000 K
done
clear
View Answer play_arrow
question_answer 39) A hollow charged metal sphere has a radius r. If the potential difference between its surface and a point at a distance 3 r from the centre is V, then the electric intensity at a distance 3 r from the centre is :
A)
\[\frac{v}{6r}\]
done
clear
B)
\[\frac{v}{4r}\]
done
clear
C)
\[\frac{V}{3r}\]
done
clear
D)
\[\frac{V}{2r}\]
done
clear
View Answer play_arrow
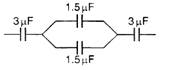
question_answer 40)
The equivalent capacitance in the following circuit is :
A)
\[2\mu F\]
done
clear
B)
\[1\mu F\]
done
clear
C)
\[1.5\mu F\]
done
clear
D)
\[3\mu F\]
done
clear
View Answer play_arrow
question_answer 41) An electrical cable of copper has just one wire of radius 9 mm. Its resistance is 10\[\Omega \]. Thus, single Cu cable is to be replaced by another cable containing 10 well insulated Cu wires each of radius 3 mm. The resistance of the new cable is:
A)
7.50\[\Omega \]
done
clear
B)
9\[\Omega \]
done
clear
C)
9.5\[\Omega \]
done
clear
D)
10\[\Omega \]
done
clear
View Answer play_arrow
question_answer 42) If the current flowing through a Cu wire of 1 mm diameter is 1.1 A. The drift velocity of electrons is:
A)
0.1 mm/s
done
clear
B)
0.2mm/s
done
clear
C)
0.3 mm/s
done
clear
D)
0.5 mm/s
done
clear
View Answer play_arrow
question_answer 43) When a 40 W lamp is connected in series with a 100 W lamp across a 210 V supply, which one glows brighter?
A)
100 W lamp
done
clear
B)
40 W lamp
done
clear
C)
Both will glow equally
done
clear
D)
It cannot be predicted
done
clear
View Answer play_arrow
question_answer 44) Which one of the following causes production of heat when current is set up in a wire?
A)
Fall of electrons from higher orbits to lower orbits
done
clear
B)
Interatomic collisions
done
clear
C)
Inter electron collisions
done
clear
D)
Collisions of conduction electrons with atoms
done
clear
View Answer play_arrow
question_answer 45) An electric charge in uniform motion produces :
A)
an electric field only
done
clear
B)
magnetic field only
done
clear
C)
both electric and magnetic field
done
clear
D)
no fields at all
done
clear
View Answer play_arrow
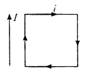
question_answer 46)
A rectangular loop carrying a current i is situated near a long straight wire such that the current carrying wire is parallel to one of the sides of a loop. If a steady current is established in a wire the loop will:
A)
rotate about an axis parallel to the wire
done
clear
B)
move away from the wire
done
clear
C)
move towards the wire
done
clear
D)
remains stationary
done
clear
View Answer play_arrow
question_answer 47) The sensitivity of a moving coil galvanometer depends on :
A)
angle of deflection
done
clear
B)
earths magnetic field
done
clear
C)
moment of inertia of the coil
done
clear
D)
none of the above
done
clear
View Answer play_arrow
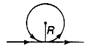
question_answer 48)
An infinite straight current carrying conductor is bent into a circle as shown in the figure. If the radius of the circle is R, the magnetic field at the centre of the coil is:
A)
infinite
done
clear
B)
zero
done
clear
C)
\[\frac{{{\mu }_{0}}2i}{4\pi R}\pi \]
done
clear
D)
\[\frac{{{\mu }_{0}}2i}{4\pi R}(\pi +1)\]
done
clear
View Answer play_arrow
question_answer 49) A proton enters a magnetic field with velocity parallel to the magnetic field. The path followed by the proton is a :
A)
circle
done
clear
B)
parabola
done
clear
C)
helix
done
clear
D)
straight line
done
clear
View Answer play_arrow
question_answer 50) In the Seebeck series bismuth occurs first followed by Cu and Fe among other. The Sb is the last in series. If \[{{V}_{1}}\] be the thermo emf at the given temperature difference for Bi-Sb thermocouple and \[{{V}_{2}}\]be that for Cu-Fe thermocouple, then :
A)
\[{{V}_{1}}=\text{ }{{V}_{2}}\]
done
clear
B)
\[{{V}_{1}}<\text{ }{{V}_{2}}\]
done
clear
C)
\[{{V}_{1}}>\text{ }{{V}_{2}}\]
done
clear
D)
data insufficient
done
clear
View Answer play_arrow
question_answer 51) Which of the following is a characteristic temperature for the thermocouple?
A)
Temperature of cold junction
done
clear
B)
Temperature of hot junction
done
clear
C)
Temperature of inversion
done
clear
D)
Neutral temperature
done
clear
View Answer play_arrow
question_answer 52) When a magnet is moved with its north pole towards a coil placed in a closed circuit, then the nearest face of the coil shows :
A)
south polarity
done
clear
B)
north polarity
done
clear
C)
no polarity
done
clear
D)
sometimes north and sometimes south polarity
done
clear
View Answer play_arrow
question_answer 53) The expression for the induced emf contains negative sign \[\left( E=\frac{-d\theta }{dt} \right).\] What is the significance of negative sign?
A)
The induced emf is produced only the negative flux decreases
done
clear
B)
The induced emf opposes the change in the magnetic flux
done
clear
C)
The induced emf is opposite to the direction of the flux
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 54) When the number of turns in a coil is doubled without any change in the length of the coil, its self-inductance becomes :
A)
4 times
done
clear
B)
2 times
done
clear
C)
halved
done
clear
D)
remains unchanged
done
clear
View Answer play_arrow
question_answer 55) A step up transformer operates on a 200 V line and supplies to a load of 2 A. The ratio of primary to secondary windings is 1:25. Determine the primary current.
A)
8.8 A
done
clear
B)
0.08 A
done
clear
C)
12.5 A
done
clear
D)
50 A
done
clear
View Answer play_arrow
question_answer 56) In an AC circuit the instantaneous values of emf and current are E = 200 sin 314t volt and\[i=\frac{1}{2}\sin \] ampere. The average power consumed in watts is:
A)
200
done
clear
B)
100
done
clear
C)
50
done
clear
D)
25
done
clear
View Answer play_arrow
question_answer 57) A series LCR circuit is tuned to resonance. The impedance of the circuit at resonance is :
A)
\[{{\left[ {{R}^{2}}+{{\left( \omega L-\frac{1}{\omega C} \right)}^{2}} \right]}^{{1}/{2}\;}}\]
done
clear
B)
\[{{\left[ {{R}^{2}}+{{(\omega L)}^{2}}+{{\left( \frac{1}{\omega C} \right)}^{2}} \right]}^{{1}/{2}\;}}\]
done
clear
C)
\[{{\left[ {{R}^{2}}+{{\left( \frac{1}{\omega C} \right)}^{2}} \right]}^{{1}/{2}\;}}\]
done
clear
D)
R
done
clear
View Answer play_arrow
question_answer 58) In a Millikan oil drop experiment an oil drop carrying a charge of 2e is kept balanced between two parallel plates 2 cm apart, when a potential difference of 12000 V is applied to them, the radius of the oil drop is : \[(\rho =2\times {{10}^{3}}kg/{{m}^{2}})\]
A)
\[1.3\times {{10}^{-6}}m\]
done
clear
B)
\[3.4\times {{10}^{-5}}m\]
done
clear
C)
\[~4\times {{10}^{-6}}m\]
done
clear
D)
\[5\times {{10}^{-6}}m\]
done
clear
View Answer play_arrow
question_answer 59) Light of wavelength 0.6 \[\mu \]m from a sodium lamp falls on a photocell and causes the emission of photoelectrons for which the stopping potential is 0.5 V. With light of wavelength 0.4 \[\mu \]m from a sodium lamp, the stopping potential is 1.5 V. With this data, the value of \[\frac{h}{e}\] is :
A)
\[4\times {{10}^{-59}}Vs\]
done
clear
B)
\[0.25\times {{10}^{55}}Vs\]
done
clear
C)
\[4\times {{10}^{-15}}Vs\]
done
clear
D)
\[4\times {{10}^{-8}}Vs\]
done
clear
View Answer play_arrow
question_answer 60) In photoelectric effect, the number of photoelectrons emitted is proportional to :
A)
intensity of incident beam
done
clear
B)
frequency of incident beam
done
clear
C)
velocity of incident beam
done
clear
D)
work function of photocathode
done
clear
View Answer play_arrow
question_answer 61) The ratio of radii of nuclei \[_{13}^{27}\]Al and \[_{52}^{152}Te\] is approximately:
A)
6 : 10
done
clear
B)
13 : 52
done
clear
C)
40 : 177
done
clear
D)
14 : 7
done
clear
View Answer play_arrow
question_answer 62) A source of light is placed at a distance of 1 m from a photocell and the cut-off potential is found to be V0. If the lamp is now placed at a distance of 2 m, the cut-off potential will become :
A)
\[2{{V}_{0}}\]
done
clear
B)
\[\frac{{{V}_{0}}}{2}\]
done
clear
C)
\[\frac{{{V}_{0}}}{4}\]
done
clear
D)
\[{{V}_{0}}\]
done
clear
View Answer play_arrow
question_answer 63) According to Bohr theory the radius of electron orbit described by principal atomic number n and for atomic number Z is proportional:
A)
\[{{Z}^{2}}{{n}^{2}}\]
done
clear
B)
\[\frac{{{Z}^{2}}}{{{n}^{2}}}\]
done
clear
C)
\[\frac{{{n}^{2}}}{Z}\]
done
clear
D)
\[\frac{{{Z}^{2}}}{n}\]
done
clear
View Answer play_arrow
question_answer 64) According to Bohrs postulate which of the following take discrete value?
A)
Kinetic energy
done
clear
B)
Potential energy
done
clear
C)
Angular momentum
done
clear
D)
Linear momentum
done
clear
View Answer play_arrow
question_answer 65) If elements with principal quantum number n > 4 were not allowed in nature, the number of possible elements would be :
A)
60
done
clear
B)
32
done
clear
C)
34
done
clear
D)
64
done
clear
View Answer play_arrow
question_answer 66) The nucleus of an atom consists of :
A)
electrons and protons
done
clear
B)
electrons, protons and neutrons
done
clear
C)
electrons and neutrons
done
clear
D)
neutrons and protons
done
clear
View Answer play_arrow
question_answer 67) Which of the following is not correct? In Bohr model of hydrogen atom :
A)
the radius of nth orbit is proportional to n2
done
clear
B)
the total energy of electron in nth orbit is proportional to n
done
clear
C)
the angular momentum of an electron in an orbit is an integral multiple of \[\frac{h}{2\pi }\]
done
clear
D)
the magnitude of the potential energy of an electron in any orbit is greater than its kinetic energy
done
clear
View Answer play_arrow
question_answer 68) 1 mg of radius has \[2.68\times {{10}^{18}}\] nuclei. Its half-life is 1620 yr. After 3240 yr, how many nuclei would have disintegrated?
A)
\[1.82\times {{10}^{18}}\]
done
clear
B)
\[0.67\times {{10}^{18}}\]
done
clear
C)
\[1.34\times {{10}^{18}}\]
done
clear
D)
\[2.01\times {{10}^{11}}\]
done
clear
View Answer play_arrow
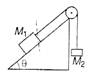
question_answer 69)
Two masses \[{{M}_{1}}\]and \[{{M}_{2}}\] are attached to the ends of a string which passes over a pulley attached to the top of an inclined plane. The angle of inclination of the plane is, 30° and \[{{M}_{1}}=10\text{ }kg,{{M}_{2}}=5\text{ }kg.\] What is the acceleration of mass \[{{M}_{2}}\]?
A)
\[10m/{{s}^{2}}~\]
done
clear
B)
\[~5m/{{s}^{2}}\]
done
clear
C)
\[\frac{2}{3}m/{{s}^{2}}\]
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 70) A choke coil is used in :
A)
AC circuit only
done
clear
B)
DC circuit only
done
clear
C)
AC and DC circuit
done
clear
D)
neither AC nor DC circuit
done
clear
View Answer play_arrow
question_answer 71) The unit of molar gas constant is :
A)
\[J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
B)
J
done
clear
C)
JK
done
clear
D)
\[Jmo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 72) The angle of projection for which the horizontal range is equal to the maximum height of a projectile is :
A)
\[\theta ={{\tan }^{-1}}(1)\]
done
clear
B)
\[\theta ={{\tan }^{-1}}\](4)
done
clear
C)
\[\theta ={{\tan }^{-1}}\](3)
done
clear
D)
\[\theta ={{\tan }^{-1}}\](2)
done
clear
View Answer play_arrow
question_answer 73) A cyclist covers a circular path 34.3 cm long in \[\sqrt{22}s.\] The angle of inclination of the cyclist is : (Given, \[g=9.8\text{ }m/{{s}^{2}}\])
A)
\[50{}^\circ C\]
done
clear
B)
\[45{}^\circ C\]
done
clear
C)
\[30{}^\circ C\]
done
clear
D)
\[60{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 74) Radius of gyration of a body depends on :
A)
mass and size of body
done
clear
B)
mass distribution and axis of rotation
done
clear
C)
size of body
done
clear
D)
mass of body
done
clear
View Answer play_arrow
question_answer 75) The moment of inertia of a semicircular ring about its centre is :
A)
\[M{{R}^{2}}\]
done
clear
B)
\[\frac{M{{R}^{2}}}{2}\]
done
clear
C)
\[\frac{M{{R}^{2}}}{4}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 76) Anomalous behaviour of nitrogen is due to:
A)
small size and high electronegativity
done
clear
B)
Non-availability of d-orbitals in the valence shell
done
clear
C)
ease of multiple bond formation
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 77) Which of the following cations has the maximum number of unpaired electrons?
A)
\[M{{n}^{2+}}\]
done
clear
B)
\[F{{e}^{2+}}\]
done
clear
C)
\[C{{o}^{2+}}\]
done
clear
D)
\[N{{i}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 78) Which of the following transition metal ion is not coloured?
A)
\[C{{u}^{2+}}\]
done
clear
B)
\[{{V}^{3+}}\]
done
clear
C)
\[C{{o}^{2+}}\]
done
clear
D)
\[N{{i}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 79) For the process: \[C{{O}_{2}}(s)\xrightarrow{{}}C{{O}_{2}}(s)\]
A)
both\[\Delta H\]and\[\Delta S\]are +ve.
done
clear
B)
\[\Delta H\]is -ve and\[\Delta S\]is +ve
done
clear
C)
\[\Delta H\]is +ve and\[\Delta S\]is\[-ve\]
done
clear
D)
both\[\Delta H\]and\[\Delta S\]are\[-ve\]
done
clear
View Answer play_arrow
question_answer 80) Gel is a system of a:
A)
solid dispersed in liquid medium
done
clear
B)
solid dissolved in gaseous medium
done
clear
C)
liquid dissolved in liquid medium
done
clear
D)
liquid dispersed in solid medium
done
clear
View Answer play_arrow
question_answer 81) For adsorption of gas in solid,\[\log \left( \frac{x}{m} \right)\]versus \[log\text{ }P\]is linear with the slope equal to:
A)
\[K\]
done
clear
B)
\[log\,K\]
done
clear
C)
\[n\]
done
clear
D)
\[\frac{1}{n}\]
done
clear
View Answer play_arrow
question_answer 82) Osteomalacia in adults are produced by the deficiency of vitamin:
A)
\[{{B}_{6}}\]
done
clear
B)
\[H\]
done
clear
C)
\[D\]
done
clear
D)
\[E\]
done
clear
View Answer play_arrow
question_answer 83) Protein that controls the metabolism of glucose:
A)
oxytocin
done
clear
B)
insulin
done
clear
C)
keratin
done
clear
D)
trypsin
done
clear
View Answer play_arrow
question_answer 84) Which of the following is not present in RNA?
A)
Uracil
done
clear
B)
Phosphate
done
clear
C)
Thymine
done
clear
D)
Ribose
done
clear
View Answer play_arrow
question_answer 85) Bakelite is obtained from phenol by reaction with:
A)
acetaldehyde
done
clear
B)
acetal
done
clear
C)
formaldehyde
done
clear
D)
chlorobenzene
done
clear
View Answer play_arrow
question_answer 86) Terylene is a polymer produced by condensation of ethylene glycol with:
A)
succinic acid
done
clear
B)
terephthalic acid
done
clear
C)
oxalic acid
done
clear
D)
adipic acid
done
clear
View Answer play_arrow
question_answer 87) Leibermanns nitroso reaction may be used as a test for:
A)
\[{{1}^{o}}\]amine
done
clear
B)
\[{{2}^{o}}\]amine
done
clear
C)
\[{{3}^{o}}\]amine
done
clear
D)
quaternary amine
done
clear
View Answer play_arrow
question_answer 88) Nitromethane reacts with chlorine in the presence of a base to give:
A)
chloroform
done
clear
B)
chloropicrin
done
clear
C)
nitrosyi chloride
done
clear
D)
chloromethane
done
clear
View Answer play_arrow
question_answer 89) Which of the following will not undergo Hell-Volhard-Zelinsky reaction?
A)
Acetic acid
done
clear
B)
Formic acid
done
clear
C)
Propionic acid
done
clear
D)
2-methyl propionic acid
done
clear
View Answer play_arrow
question_answer 90) The bond formed between carbon atom 1 and carbon atom 2 in compound \[\underset{1}{\mathop{C}}\,{{H}_{2}}=\underset{2}{\mathop{C}}\,=\underset{3}{\mathop{C}}\,H\underset{4}{\mathop{C}}\,{{H}_{3}}\]involves the hybrids as:
A)
\[sp\]and\[sp\]
done
clear
B)
\[s{{p}^{3}}\] and\[sp\]
done
clear
C)
\[s{{p}^{2}}\]and\[sp\]
done
clear
D)
\[sp\]and\[s{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 91) Roasting is the process of heating the concentrated ore strongly:
A)
in the presence of excess of air
done
clear
B)
in the presence of limited supply of air
done
clear
C)
pin the absence of air
done
clear
D)
in the presence of nitrogen
done
clear
View Answer play_arrow
question_answer 92) The ore horn silver corresponds to:
A)
\[AgCl\]
done
clear
B)
\[AgBr\]
done
clear
C)
\[A{{g}_{2}}S\]
done
clear
D)
\[3A{{g}_{2}}S.S{{b}_{2}}{{S}_{3}}\]
done
clear
View Answer play_arrow
question_answer 93) German silver is an alloy of:
A)
\[Cu+Zn+Ni\]
done
clear
B)
\[Ag+Cu+Zn\]
done
clear
C)
Cu and Zn
done
clear
D)
Ag and Ni
done
clear
View Answer play_arrow
question_answer 94) IUPAC name of the compound \[C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,\underset{\begin{smallmatrix} | \\ C{{H}_{2}}-{{(C{{H}_{2}})}_{3}}-C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]is:
A)
6,7-dimethyl, 1-7-n-propylnonane
done
clear
B)
4,5-dimethyl, 1-4-ethyldecane
done
clear
C)
3,4-dimethyl-4-n-propylnonane
done
clear
D)
6,7-dimethyl, 1-7-ethyldecane
done
clear
View Answer play_arrow
question_answer 95) Which of the following is the correct statement for\[{{C}_{4}}H\]?
A)
It must have a double bond
done
clear
B)
It must have a triple bond
done
clear
C)
It should not have either a double or triple bond
done
clear
D)
Such a compound is impossible
done
clear
View Answer play_arrow
question_answer 96) The number of sigma and pi-bonds in but-1-en-3-yne are:
A)
5 sigma and 5 pi
done
clear
B)
8 sigma and 3 pi
done
clear
C)
8 sigma and 2 pi
done
clear
D)
7 sigma and 2 pi
done
clear
View Answer play_arrow
question_answer 97) Which of the following pairs represent stereoisomerism?
A)
Geometrical and position isomerism
done
clear
B)
Geometrical and conformational isomerism
done
clear
C)
Optical and functional isomerism
done
clear
D)
Metamerism and optical isomerism
done
clear
View Answer play_arrow
question_answer 98) Vinyl alcohol and acetaldehyde are:
A)
chain isomers
done
clear
B)
keto-enol tautomers
done
clear
C)
geometrical isomers
done
clear
D)
position isomers
done
clear
View Answer play_arrow
question_answer 99) A fuel containing 25% n-heptane and 75% iso-octane has got octane number:
A)
25
done
clear
B)
50
done
clear
C)
75
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 100) Lindlars catalyst is:
A)
\[Pd\]and\[C{{O}_{2}}\]
done
clear
B)
\[Pd\]and\[{{H}_{2}}\]
done
clear
C)
palladised charcoal deactivated with sulphur
done
clear
D)
\[Pd\]and\[CaC{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 101) The reaction of phenol with alkaline chloroform gives salicylaldehyde, it is called:
A)
Gattermann aldehyde synthesis
done
clear
B)
Perkins reaction
done
clear
C)
Reimer-Tiemann reaction
done
clear
D)
Duff reaction
done
clear
View Answer play_arrow
question_answer 102) If chloroform is left open in the presence of sunlight, then:
A)
polymerisation takes place
done
clear
B)
explosion takes place
done
clear
C)
poisonous phosgene gas is formed
done
clear
D)
no reaction takes place
done
clear
View Answer play_arrow
question_answer 103) Which of the following property is not associated with glycerol?
A)
Formation of tartronic acid on oxidation
done
clear
B)
Formation of acrolein on dehydration
done
clear
C)
Formation of\[{{H}_{2}}O,\text{ }C{{O}_{2}}\]on reduction
done
clear
D)
Formation of allyl iodide with\[HI\]
done
clear
View Answer play_arrow
question_answer 104) Which of the following is obtained when sodium phenoxide is heated with ethyl iodide?
A)
Phenetole
done
clear
B)
Phenol
done
clear
C)
Ethyl phenyl alcohol
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 105) Which of the following statements is false in case of epoxides?
A)
Epoxides are very reactive compounds due to the case of opening of highly strained three membered ring
done
clear
B)
The \[C-O\] bonds in the epoxides are weaker than in ordinary ethers
done
clear
C)
Epoxides are less stable than aliphatic ether molecule
done
clear
D)
Epoxides are inert as compared to ethers
done
clear
View Answer play_arrow
question_answer 106) Formalin is a:
A)
100% solution of \[HCHO\]
done
clear
B)
40% solution of\[HCHO\]
done
clear
C)
60% solution of\[C{{H}_{3}}CHO\]
done
clear
D)
40% solution of\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 107) Which of the following is the least pure form of iron?
A)
Bessemer iron
done
clear
B)
Pig iron
done
clear
C)
Wrought iron
done
clear
D)
Steel iron
done
clear
View Answer play_arrow
question_answer 108) Washing soda has the formula:
A)
\[N{{a}_{2}}C{{O}_{3}}-7{{H}_{2}}O\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}-10{{H}_{2}}O\]
done
clear
C)
\[Na{{ }_{2}}C{{O}_{3}}-{{H}_{2}}O\]
done
clear
D)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 109) The electronic configuration of chalcogens is:
A)
\[n{{s}^{2}}\text{ }n{{p}^{2}}\]
done
clear
B)
\[n{{s}^{2}}\text{ }n{{p}^{3}}\]
done
clear
C)
\[n{{s}^{2}}\text{ }n{{p}^{4}}\]
done
clear
D)
\[n{{s}^{2}}\text{ }n{{p}^{1}}\]
done
clear
View Answer play_arrow
question_answer 110) The preparation ofethylacetoacetate involves:
A)
Reformatsky reaction
done
clear
B)
Claisen condensation
done
clear
C)
Cannizaros reaction
done
clear
D)
Wittig reaction
done
clear
View Answer play_arrow
question_answer 111) Acetamide on heating with\[{{P}_{4}}{{O}_{10}}\]gives:
A)
\[C{{H}_{3}}NC\]
done
clear
B)
\[C{{H}_{3}}CN\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
\[{{(C{{H}_{3}}CO)}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 112) Neutron is a fundamental particle carrying:
A)
a charge of\[+1\]unit and a mass of one unit
done
clear
B)
no charge and a mass of one unit
done
clear
C)
no charge and no mass
done
clear
D)
a charge of\[-1\]unit and no mass
done
clear
View Answer play_arrow
question_answer 113) Which of the following rays are highly toxic to living being?
A)
\[\gamma -\]rays
done
clear
B)
\[\beta -\]rays
done
clear
C)
\[\alpha -\]rays
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 114) The equilibrium will be shifted in the opposite direction if: \[PC{{l}_{3}}(g)+C{{l}_{2}}(g)PC{{l}_{5}}(g)\]
A)
\[PC{{l}_{3}}\]is added
done
clear
B)
helium gas is added
done
clear
C)
catalyst is added
done
clear
D)
pressure is reduced
done
clear
View Answer play_arrow
question_answer 115) If pH value of 0.1 mol/L\[HCl\] is nearly 1, then pH value of 0.05 mol/L\[{{H}_{2}}S{{O}_{4}}\]is:
A)
0.5
done
clear
B)
1.0
done
clear
C)
0.05
done
clear
D)
2.00
done
clear
View Answer play_arrow
question_answer 116) Blood cells retain their shape in solution which are:
A)
isotonic to blood
done
clear
B)
hypotonic to blood
done
clear
C)
hypertonic to blood
done
clear
D)
equinormal to blood
done
clear
View Answer play_arrow
question_answer 117) The mixture of n-hexane and n-heptane is an example of:
A)
real solution
done
clear
B)
dilute solution
done
clear
C)
ideal solution
done
clear
D)
non-real solution
done
clear
View Answer play_arrow
question_answer 118) Equal volumes of molar hydrochloric acid and sulphuric acid are neutralized by dilute\[NaOH\]solution and\[x\]kcals and y kcals of heat are liberated respectively. Which of the following is true?
A)
\[x=y\]
done
clear
B)
\[x=\frac{1}{2}y\]
done
clear
C)
\[x=2y\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 119) Which of the following organometallic compound is\[\sigma \]and\[\pi -\]bonded?
A)
\[[Fe{{({{\eta }^{5}}-{{C}_{2}}{{H}_{5}})}_{2}}]\]
done
clear
B)
\[[PtC{{l}_{3}}({{\eta }^{2}}-{{C}_{2}}{{H}_{4}})]\]
done
clear
C)
\[{{[Co{{(CO)}_{5}}N{{H}_{3}}]}^{2+}}\]
done
clear
D)
\[Al{{(C{{H}_{3}})}_{3}}\]
done
clear
View Answer play_arrow
question_answer 120) How many coulombs of electricity are consumed when 100 mA current is passed through a solution of\[AgN{{O}_{3}}\]for half an hour during an electrolysis experiment?
A)
108
done
clear
B)
18000
done
clear
C)
180
done
clear
D)
3000
done
clear
View Answer play_arrow
question_answer 121) \[{{E}^{o}}\]value of\[M{{g}^{2+}}/Mg,F{{e}^{2+}}/Fe\]and \[Z{{n}^{2+}}/Zn\]are\[-2.37\text{ }V,-0.44\text{ }V\]and\[-\,0.76\text{ }V\] respectively. The correct statement is:
A)
Mg oxidises Fe
done
clear
B)
Zn oxidises Fe
done
clear
C)
Zn reduces\[M{{g}^{2+}}\]
done
clear
D)
Zn reduces\[F{{e}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 122) The behaviour of a real gas approaches that of an ideal gas at:
A)
high temperature and low pressure
done
clear
B)
low temperature and high pressure
done
clear
C)
\[{{0}^{o}}C\]and 760 mm of Hg
done
clear
D)
\[{{100}^{o}}C\] and 760 mm of Hg
done
clear
View Answer play_arrow
question_answer 123) The first ionization potential of\[Na,Mg,\text{ }Al\]and\[Si\]are in order:
A)
\[Na<Mg>Al<Si\]
done
clear
B)
\[Na>Mg>Al>Si\]
done
clear
C)
\[Na<Mg<Al<Si\]
done
clear
D)
\[Na>Mg>Al<Si\]
done
clear
View Answer play_arrow
question_answer 124) Of the metals\[Be,\text{ }Mg,\text{ }Ca\]and Sr of group 2 in the periodic table, the least ionic chloride will be formed by:
A)
Be
done
clear
B)
Ca
done
clear
C)
Mg
done
clear
D)
Sr
done
clear
View Answer play_arrow
question_answer 125) The highest oxidation state achieved by transition metal is given by:
A)
ns electrons
done
clear
B)
\[(n-1)d\] electrons
done
clear
C)
\[(n+1)d\] electrons
done
clear
D)
\[ns(n-1)d\] electrons
done
clear
View Answer play_arrow
question_answer 126) Which of the following compounds exhibit co-ordination isomerism?
A)
\[[Co{{(N{{H}_{3}})}_{5}}S{{O}_{4}}]Cl\]
done
clear
B)
\[[Co{{(N{{H}_{3}})}_{6}}][Cr{{(CN)}_{6}}]\]
done
clear
C)
\[[Co{{(e{{n}_{2}})}_{2}}]{{[N{{O}_{2}}]}_{3}}\]
done
clear
D)
\[[Cr{{({{H}_{2}}O)}_{6}}]C{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 127) The oxidation number of platinum in\[{{[Pt({{C}_{2}}{{H}_{4}})C{{l}_{3}}]}^{-}}\]is:
A)
+1
done
clear
B)
+2
done
clear
C)
+3
done
clear
D)
+4
done
clear
View Answer play_arrow
question_answer 128) Gold number is an index for:
A)
amount of gold present in colloid
done
clear
B)
amount of gold required to present the colloid
done
clear
C)
amount of gold required to break the colloid
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 129) The predominant intermolecular forces in HF is due to:
A)
dipole-induced dipole interaction
done
clear
B)
hydrogen bond formation
done
clear
C)
dispersion interaction
done
clear
D)
dipole-dipole interaction
done
clear
View Answer play_arrow
question_answer 130)
At 298 K, the standard reduction potentials for the following half reactions are given as: \[Z{{n}^{2+}}+2{{e}^{}}\xrightarrow{{}}Zn(s)\] \[\,0.762\] \[C{{r}^{3+}}(aq)+3{{e}^{}}\xrightarrow{{}}Cr(s)\] \[\,0.740\] \[2{{H}^{+}}(aq)+2{{e}^{}}\xrightarrow{{}}{{H}_{2}}(g)\] \[0.00\] \[F{{e}^{3+}}(aq)+3{{e}^{}}\xrightarrow{{}}Fe(aq)\] \[+\,0.770\]
The strongest reducing agent is:
A)
Zn (s)
done
clear
B)
H (s)
done
clear
C)
Cr (s)
done
clear
D)
Fe (s)
done
clear
View Answer play_arrow
question_answer 131) Transition from\[n=4,5,6\]to\[n=3\]in hydrogen spectrum gives:
A)
Lyman series
done
clear
B)
Balmer series
done
clear
C)
Paschen series
done
clear
D)
P fund series
done
clear
View Answer play_arrow
question_answer 132) 2.0 g of oxygen contains number of atoms equal to that in:
A)
4.0 g of sulphur
done
clear
B)
7.0 g of nitrogen
done
clear
C)
0.5 g of hydrogen
done
clear
D)
2.3 got sodium
done
clear
View Answer play_arrow
question_answer 133) An element\[X\]loses\[1\,\alpha \]and\[2\beta -\] particles in three successive stages. The resulting element will be:
A)
an isobar of X
done
clear
B)
an isotope of X
done
clear
C)
an isotone of X
done
clear
D)
X itself
done
clear
View Answer play_arrow
question_answer 134) The reaction\[A\xrightarrow{{}}B\]follows a second order kinetics. Doubling the concentration of A will increase the rate of formation of B by a factor of:
A)
2
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
4
done
clear
D)
\[\frac{1}{4}\]
done
clear
View Answer play_arrow
question_answer 135) In an exothermic reaction at\[{{10}^{o}}C\]rise in temperature will:
A)
decrease the value of equilibrium constant
done
clear
B)
double the value of\[{{K}_{c}}\]
done
clear
C)
do not produce any change in\[{{K}_{c}}\]
done
clear
D)
produce some change in\[{{K}_{c}}\]
done
clear
View Answer play_arrow
question_answer 136) Number of molecules in one litre of water is close to:
A)
\[55.5\times 6.023\times {{10}^{23}}\]
done
clear
B)
\[18\times 6.023\times {{10}^{23}}\]
done
clear
C)
\[18\div 22.4\times {{10}^{23}}\]
done
clear
D)
\[1.8\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 137) The change in heat energy of a chemical reaction at constant temperature and pressure is called:
A)
enthalpy
done
clear
B)
free energy
done
clear
C)
internal energy
done
clear
D)
bond energy
done
clear
View Answer play_arrow
question_answer 138) Which of the following methods of expressing the concentration is independent of temperature?
A)
Molarity
done
clear
B)
Molality
done
clear
C)
Normality
done
clear
D)
Formality
done
clear
View Answer play_arrow
question_answer 139) Which of the following solutions would produce maximum elevation in boiling point?
A)
0.1 M\[BaC{{l}_{2}}\]
done
clear
B)
\[0.1\text{ }M\text{ }KCl\]
done
clear
C)
0.1 M glucose
done
clear
D)
0.1 M sucrose
done
clear
View Answer play_arrow
question_answer 140) When\[HCl\]gas is passed through a saturated solution of\[NaCl\](brine), then the solubility of \[NaCl\]:
A)
increases
done
clear
B)
decreases
done
clear
C)
remains same
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 141) If the concentration is expressed in mole per litre, the unit of rate constant for a first order reaction is:
A)
\[mol\,{{L}^{-1}}\]
done
clear
B)
\[L\text{ }mo{{l}^{-1}}\]
done
clear
C)
\[{{s}^{-1}}\]
done
clear
D)
\[mol\,{{L}^{-1}}\,{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 142) Hesss law is used for the determination of:
A)
heat of formation
done
clear
B)
heat of reaction
done
clear
C)
heat of transition
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 143) Which of the following is a polar molecule?
A)
\[C{{O}_{2}}\]
done
clear
B)
\[CHC{{l}_{3}}\]
done
clear
C)
\[CC{{l}_{4}}\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 144) Each unit cell of\[NaCl\]consists of 6 chlorine atoms and:
A)
13 Na atoms
done
clear
B)
14 Na atoms
done
clear
C)
8 Na atoms
done
clear
D)
6 Na atoms
done
clear
View Answer play_arrow
question_answer 145) Five grams of the following gas at\[{{87}^{o}}C\]and 750 mm pressure is taken. The gas with least volume is:
A)
\[HF\]
done
clear
B)
\[HCl\]
done
clear
C)
\[HBr\]
done
clear
D)
\[HI\]
done
clear
View Answer play_arrow
question_answer 146) \[C{{u}^{+}}\]ion is not stable in aqueous solution because of disproportionation reaction.\[{{E}^{o}}\] value for disproportionation of\[C{{u}^{+}}\]is: (Given: \[E_{C{{u}^{2+}}|C{{u}^{+}}}^{o}=0.15V,\]\[E_{C{{u}^{2+}}|Cu}^{o}=0.34\,V\])
A)
\[-\,0.19V\]
done
clear
B)
0.38
done
clear
C)
0.94V
done
clear
D)
\[-\,0.38V\]
done
clear
View Answer play_arrow
question_answer 147) The predominant product formed when 3-methyl-2-pentene reacts with\[HOCl\]is:
A)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,-\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,-\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{C}}}\,-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 148) Which of the following species is least stable?
A)
\[P-{{O}_{2}}N-{{C}_{6}}{{H}_{4}}{{-}^{+}}C{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}{{-}^{+}}C{{H}_{2}}\]
done
clear
C)
\[P-Cl-{{C}_{6}}{{H}_{4}}{{-}^{+}}C{{H}_{2}}\]
done
clear
D)
\[P-C{{H}_{3}}O-{{C}_{6}}{{H}_{4}}{{-}^{+}}C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 149) Among the following species, identify the isostructural pairs: \[N{{F}_{3}},\text{ }NO_{3}^{-},\text{ }B{{F}_{3}},\text{ }{{H}_{3}}{{O}^{+}},\text{ }H{{N}_{3}}\]
A)
\[[N{{F}_{3}},NO_{3}^{-}]\]and\[[B{{F}_{3}},{{H}_{3}}{{O}^{+}}]\]
done
clear
B)
\[[N{{F}_{3}},H{{N}_{3}}]\]and \[[NO_{3}^{-},B{{F}_{3}}]\]
done
clear
C)
\[[N{{F}_{3}},{{H}_{3}}{{O}^{+}}]\]and \[[NO_{3}^{-},B{{F}_{3}}]\]
done
clear
D)
\[[N{{F}_{3}},{{H}_{3}}{{O}^{+}}]\]and\[[H{{N}_{3}},B{{F}_{3}}]\]
done
clear
View Answer play_arrow
question_answer 150) Which of the following metal ions plays an important role in the muscle contraction?
A)
\[N{{a}^{+}}\]
done
clear
B)
\[{{K}^{+}}\]
done
clear
C)
\[F{{e}^{3+}}\]
done
clear
D)
\[C{{a}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 151) Golgi apparatus is found in:
A)
prokaryotic cell
done
clear
B)
eukaryotic cell
done
clear
C)
mate gametes of bryophytes
done
clear
D)
near mitochondria
done
clear
View Answer play_arrow
question_answer 152) Status of a foetus in the womb can be studied by:
A)
simple X-ray
done
clear
B)
ultrasound scanning
done
clear
C)
only by operation
done
clear
D)
barium X-ray
done
clear
View Answer play_arrow
question_answer 153) Both oxysomes and quantasomes are found in:
A)
chloroplasts
done
clear
B)
mitocliondria
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 154) Binomial system of classification was given by:
A)
Charles Darwin
done
clear
B)
Robert Darwin
done
clear
C)
Robert Hooke
done
clear
D)
Carolus Linnaeus
done
clear
View Answer play_arrow
question_answer 155) Which of the following is a prokaryote?
A)
Nostoc
done
clear
B)
Protozoa
done
clear
C)
Prosopis
done
clear
D)
Chlorella
done
clear
View Answer play_arrow
question_answer 156) Antibodies are:
A)
foreign bodies in all cells
done
clear
B)
anti cancerous drugs
done
clear
C)
antibiotics
done
clear
D)
molecules synthesized by the < all on introduction of foreign bodies
done
clear
View Answer play_arrow
question_answer 157) Calcitonin is produced in:
A)
thyroid
done
clear
B)
pituitary glands
done
clear
C)
spleen
done
clear
D)
pancreas
done
clear
View Answer play_arrow
question_answer 158) Follicle Stimulating Hormones (FSH) is produced in:
A)
anterior pituitary
done
clear
B)
posterior pituitary
done
clear
C)
thyroid
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 159) Palaemon (prawn) is a :
A)
fish
done
clear
B)
insect
done
clear
C)
soft shell mollusk
done
clear
D)
crustacean
done
clear
View Answer play_arrow
question_answer 160) Apophysis is found in :
A)
mosses
done
clear
B)
Marchantia
done
clear
C)
Pteridium
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 161) A leaf is identified by :
A)
flat green lamina
done
clear
B)
presence of axillary buds
done
clear
C)
presence of leaf blade and petiole
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 162) Animals mostly do not synthesize :
A)
glycogen
done
clear
B)
proteins
done
clear
C)
vitamins
done
clear
D)
phospholipids
done
clear
View Answer play_arrow
question_answer 163) The pH of gastric juice is :
A)
2.0-3.0
done
clear
B)
2-66-6.8
done
clear
C)
3,75-8.5
done
clear
D)
4.70-9.0
done
clear
View Answer play_arrow
question_answer 164) Annulus is found in :
A)
mosses only
done
clear
B)
mosses and ferns
done
clear
C)
annual flowering plants
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 165) An underground specialized bud with reduced disk like stem, covered with fleshy leaves is called :
A)
bulb
done
clear
B)
rhizome
done
clear
C)
bulbil
done
clear
D)
rhizophore
done
clear
View Answer play_arrow
question_answer 166) Casparian scrips are found on :
A)
walls of epidermis
done
clear
B)
longitudinal walls of xylem
done
clear
C)
all walls of endodermis
done
clear
D)
radial walls of endodermis
done
clear
View Answer play_arrow
question_answer 167) Taste buds on [he posterior side of tongue in humans recognize:
A)
sweet taste
done
clear
B)
bitter taste
done
clear
C)
sour taste
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 168) Xerophthalmia is caused due to deficiency of:
A)
calcium
done
clear
B)
vitamin-A
done
clear
C)
vitamin-B
done
clear
D)
iron
done
clear
View Answer play_arrow
question_answer 169) Drug morphine is derived from :
A)
Papaver sonmifurum
done
clear
B)
Cocus nucifera
done
clear
C)
it is a synthetic drug
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 170) Endosperm in angiosperms is:
A)
tetraploid structure
done
clear
B)
triploid structure
done
clear
C)
haploid
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 171) Photottopic curvature mavena coleoptile is the result of uneven distribution of:
A)
phyiochrome
done
clear
B)
auxin
done
clear
C)
gibberellins
done
clear
D)
starch
done
clear
View Answer play_arrow
question_answer 172) Formation of m-RNA on a DNA template, is called:
A)
transcription
done
clear
B)
translation
done
clear
C)
transduction
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 173) Bacieriophages are:
A)
bacteria found in phagus
done
clear
B)
bacteria found in Phaseolus sp.
done
clear
C)
viruses infecting blue-green algae
done
clear
D)
viruses infecting bacteria
done
clear
View Answer play_arrow
question_answer 174) Which of the following is not a \[{{C}_{4}}\] plant?
A)
Maize
done
clear
B)
Crotolaria
done
clear
C)
Sorghum
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 175) Pappus is a structure characteristic of family:
A)
Asteraceae
done
clear
B)
Papaveraceae
done
clear
C)
Leguminosae
done
clear
D)
Papilionaceae
done
clear
View Answer play_arrow
question_answer 176) Which of the following is a non-communicable disease?
A)
Tuberculosis
done
clear
B)
Arthritis
done
clear
C)
Rabies
done
clear
D)
Diphtheria
done
clear
View Answer play_arrow
question_answer 177) Select the viral disease :
A)
poliomyelitis
done
clear
B)
leprosy
done
clear
C)
tetanus
done
clear
D)
diabetes
done
clear
View Answer play_arrow
question_answer 178) Auxin was First named by :
A)
Alexopoius
done
clear
B)
F.W. Went
done
clear
C)
K.V. Thimman
done
clear
D)
C. Darwin
done
clear
View Answer play_arrow
question_answer 179) Process of vernalization denotes:
A)
venation in leaves
done
clear
B)
cold treatments of seeds
done
clear
C)
vernacular names of plants
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 180) A molecule of DNA as found in chromosomes is a:
A)
double strand of many nucleotides
done
clear
B)
single strand of many nucleotides
done
clear
C)
double strand of purities and pyrimi dines
done
clear
D)
double strand of nucleosides
done
clear
View Answer play_arrow
question_answer 181) Downs syndrome is caused due to an extra chromosome in :
A)
12th chromosome
done
clear
B)
21st chromosome
done
clear
C)
sex chromosomes
done
clear
D)
2nd chromosomes
done
clear
View Answer play_arrow
question_answer 182) The full form of AIDS is:
A)
Acquired Immuno Deficiency System
done
clear
B)
Acquired Immuno Deficiency Syndrome
done
clear
C)
Assisted Immuno Deficiency Syndrome
done
clear
D)
Acquired Immuno Development Syndrome
done
clear
View Answer play_arrow
question_answer 183) Tubectomy is a method used in population control. It can be performed in :
A)
lemales
done
clear
B)
males
done
clear
C)
both (a) and (b)
done
clear
D)
in pregnant females
done
clear
View Answer play_arrow
question_answer 184) Palmella stage is found during reproduction in:
A)
Cmamydomonas
done
clear
B)
Oscitlatoria
done
clear
C)
Rhizopus
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 185) Saffaron or kesar is extracted from:
A)
entire carpel
done
clear
B)
seeds and calyx
done
clear
C)
stamens and petals
done
clear
D)
top of the style and stigma
done
clear
View Answer play_arrow
question_answer 186) The rate, of transpiration would be maximum when:
A)
both soil and air are dry
done
clear
B)
soil is dry and air is humid
done
clear
C)
soil is wet and air is humid
done
clear
D)
soil is wet and air is dry
done
clear
View Answer play_arrow
question_answer 187) Chlorofluro carbons are air polluting agents, these are produced by :
A)
diesel trucks
done
clear
B)
jet planes
done
clear
C)
rice field
done
clear
D)
acid batteries
done
clear
View Answer play_arrow
question_answer 188) Which of the following produces least of greenhouse effect?
A)
Water vapour
done
clear
B)
Carbon dioxide
done
clear
C)
Hydrogen gas
done
clear
D)
Ozone
done
clear
View Answer play_arrow
question_answer 189) In piped water supply system water is often treated with chlorine to :
A)
remove hardness
done
clear
B)
kill germs
done
clear
C)
increase oxygen level
done
clear
D)
remove algae
done
clear
View Answer play_arrow
question_answer 190) If one has to induce or increase rooting in a Stem cutting, which hormone should be applied?
A)
IBA
done
clear
B)
GA
done
clear
C)
Kinetin
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 191) In photosynthesis carbon dioxide is converted to carbohydrates. It is a ............. process :
A)
oxidative
done
clear
B)
reductive
done
clear
C)
catabolic
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 192) Oxygen evolved during photosynthesis comes from :
A)
carbohydrates
done
clear
B)
water
done
clear
C)
carbon dioxide
done
clear
D)
chlorophyll
done
clear
View Answer play_arrow
question_answer 193) Rh factor was discovered in :
A)
humans
done
clear
B)
chimpanzee
done
clear
C)
rats
done
clear
D)
monkeys
done
clear
View Answer play_arrow
question_answer 194) During cell division a cell plate is produced during:
A)
metaphase
done
clear
B)
telophase
done
clear
C)
cytokinesis
done
clear
D)
anaphase
done
clear
View Answer play_arrow
question_answer 195) One gene-one enzyme theory was prepared by:
A)
Khorana
done
clear
B)
Beadle and Tatum
done
clear
C)
Morgan
done
clear
D)
Pauling
done
clear
View Answer play_arrow
question_answer 196) Study of Bakanae disease of rice led to discovery of:
A)
gibberellins
done
clear
B)
auxins
done
clear
C)
bakery rice
done
clear
D)
both (a) and (b)
done
clear
View Answer play_arrow
question_answer 197) Green plants kept in light can produce ATP from glucose. This process is referred to as :
A)
oxidative phosphorylation
done
clear
B)
glycolysis
done
clear
C)
TCA cycie
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 198) Antibiotics are drugs used to one diseases caused by :
A)
viruses
done
clear
B)
fungi
done
clear
C)
Amoeba
done
clear
D)
bacteria
done
clear
View Answer play_arrow
question_answer 199) The lac operon concept of E. coli was proposed in 1961 by :
A)
Watson and Crick
done
clear
B)
H. G. Khorana
done
clear
C)
Meselson and Stahl
done
clear
D)
Jacob and Monod
done
clear
View Answer play_arrow
question_answer 200) Sickel cell anaemia is a ............... disease :
A)
hereditary
done
clear
B)
physiological
done
clear
C)
pathogenic
done
clear
D)
deficiency
done
clear
View Answer play_arrow
question_answer 201) An organism which could readily respire in absence of molecular oxygen could be a:
A)
fish
done
clear
B)
mushroom
done
clear
C)
Anabaena
done
clear
D)
saccharomyces
done
clear
View Answer play_arrow
question_answer 202) In Chlamydomonas the life cycle :
A)
haplontic
done
clear
B)
diplobiontic
done
clear
C)
isomorphic
done
clear
D)
haplodiplobionric
done
clear
View Answer play_arrow
question_answer 203) Out of the following which two methods of propagation, yield plants with identical genetic constitution: (i) cutting (ii) seed production (iii) mutations (iv) tissue cultures
A)
(i) and (ii)
done
clear
B)
(i) and (iii)
done
clear
C)
(i) and (iv)
done
clear
D)
(ii) and (iv)
done
clear
View Answer play_arrow
question_answer 204) Human contains........... number of chromosomes:
A)
23
done
clear
B)
23 pairs
done
clear
C)
22
done
clear
D)
22 pairs
done
clear
View Answer play_arrow
question_answer 205) Polyploidy in plants or animals can be produced by:
A)
colchicme
done
clear
B)
ultrasound
done
clear
C)
polypeptides
done
clear
D)
polyphenols
done
clear
View Answer play_arrow
question_answer 206) Which of the following is classified as a bio-femlizer?
A)
NPK mixture
done
clear
B)
Rhisobwm in legume root nodule
done
clear
C)
Rhizobium in farm yard manure
done
clear
D)
Green manure
done
clear
View Answer play_arrow
question_answer 207) DNA is normally found in nucleus. However it can also be found in :
A)
chloroplasts
done
clear
B)
vacuole
done
clear
C)
ribosomes
done
clear
D)
Golgi complex
done
clear
View Answer play_arrow
question_answer 208) Apiculture refers to rearing of :
A)
honey bees
done
clear
B)
fishes
done
clear
C)
silkworms
done
clear
D)
bacteria
done
clear
View Answer play_arrow
question_answer 209) Arachnida includes :
A)
in sects
done
clear
B)
spider
done
clear
C)
silver fish
done
clear
D)
Limulus
done
clear
View Answer play_arrow
question_answer 210) The thickness of a plasma membrane is :
A)
200 nm
done
clear
B)
7.5 nm
done
clear
C)
150 nm
done
clear
D)
1.0 nm
done
clear
View Answer play_arrow
question_answer 211) Heterocysts are found in :
A)
blue-green algae
done
clear
B)
red algae
done
clear
C)
green algae
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 212) Power house of the cell lies in :
A)
chloroplast
done
clear
B)
mitochondria
done
clear
C)
ribosomes
done
clear
D)
nucleus
done
clear
View Answer play_arrow
question_answer 213) Water blooms are formed by:
A)
Planktonic algae
done
clear
B)
Lemna
done
clear
C)
Rhizopus
done
clear
D)
Wolffia
done
clear
View Answer play_arrow
question_answer 214) Goblet cells of intestinal epithelium are example of:
A)
unicellular glands
done
clear
B)
striated epithelium
done
clear
C)
columnar epithelium
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 215) Iodine deficiency in human leads to a disease called :
A)
diabetes
done
clear
B)
mongolian idiots
done
clear
C)
goiter
done
clear
D)
myxoedema
done
clear
View Answer play_arrow
question_answer 216) Vitamin-C is also known as :
A)
vanillic acid
done
clear
B)
abscisic acid
done
clear
C)
ascorbic acid
done
clear
D)
tocopherol
done
clear
View Answer play_arrow
question_answer 217) Funaria grows well in :
A)
soils rich in cow dung
done
clear
B)
soils rich in horse dung
done
clear
C)
recently burnt soils rich in plant ash
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 218) Hypoglossal nerve controls the movements of:
A)
sub ear
done
clear
B)
heart
done
clear
C)
tongue
done
clear
D)
limbs
done
clear
View Answer play_arrow
question_answer 219) Which of the following organelie is common in plants and animals?
A)
Mitochondria
done
clear
B)
Chloroplast
done
clear
C)
Tonoplast
done
clear
D)
Centrioles
done
clear
View Answer play_arrow
question_answer 220) Triticum aescivi.im used in making bread is:
A)
hexaploid with 28 chromosomes
done
clear
B)
tecraploid with 42 chromosomes
done
clear
C)
hexaploid willi 42 chromosomes
done
clear
D)
cetraploid with 28 chromosomes
done
clear
View Answer play_arrow
question_answer 221) DMA does not contain which of the following?
A)
Adenine
done
clear
B)
Uracil
done
clear
C)
Thymine
done
clear
D)
Cyrosine
done
clear
View Answer play_arrow
question_answer 222) Removal of seed coat mechanically to remove dormancy is called as:
A)
scarification
done
clear
B)
stratification
done
clear
C)
geminauon
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 223) Duodenum has characteristic Brunners gland which secrete two hormones called :
A)
kinase, estrogen
done
clear
B)
secrerin, dioSecystokinm
done
clear
C)
prolacrin, parathormone
done
clear
D)
estradiol, progesterone
done
clear
View Answer play_arrow
question_answer 224) Injury of vagus nerve in human is not likely to effect:
A)
tongue movements
done
clear
B)
gastriubtestubak movements
done
clear
C)
pancreatic secretion
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 225) Synovial fluid is found in :
A)
cranial cavity
done
clear
B)
spinal cavity
done
clear
C)
immovable joints
done
clear
D)
free movable joints
done
clear
View Answer play_arrow