A) \[S{{F}_{4}}\]
B) \[Br{{F}_{5}}\]
C) \[S{{O}_{2}}\]
D) \[Xe{{F}_{4}}\]
Correct Answer: D
Solution :
The structure of \[\text{Xe}{{\text{F}}_{\text{4}}}\]is square planar with two positions occupied by lone pairs. It has \[\text{s}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}\]hybridization and two lone pairs of electrons.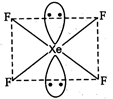
You need to login to perform this action.
You will be redirected in
3 sec
