A) elimination reaction
B) electrophilic addition reaction
C) nucleophilic substitution unimolecular reaction
D) nucleophilic substitution bimolecular reaction
Correct Answer: D
Solution :
The reaction takes place as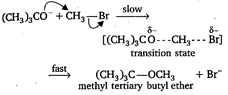 Since, in this reaction, \[\text{B}{{\text{r}}^{-}}\]is substituted by the attack of nucleophile \[{{(C{{H}_{3}})}_{3}}C{{O}^{-}},\] it is a nucleophilic substitution reaction. Moreover, the rate of the reaction depends upon the concentration of \[{{(C{{H}_{3}})}_{3}}C{{O}^{-}}\] and \[\text{C}{{\text{H}}_{\text{3}}}\text{Br}\]both, so it is a bimolecular reaction.
Since, in this reaction, \[\text{B}{{\text{r}}^{-}}\]is substituted by the attack of nucleophile \[{{(C{{H}_{3}})}_{3}}C{{O}^{-}},\] it is a nucleophilic substitution reaction. Moreover, the rate of the reaction depends upon the concentration of \[{{(C{{H}_{3}})}_{3}}C{{O}^{-}}\] and \[\text{C}{{\text{H}}_{\text{3}}}\text{Br}\]both, so it is a bimolecular reaction.
You need to login to perform this action.
You will be redirected in
3 sec
