A) tetrahedral
B) octahedral
C) square planar
D) linear
Correct Answer: B
Solution :
\[{{K}_{4}}[Fe{{(CN)}_{6}}]\rightleftharpoons 4{{K}^{+}}+{{[Fe{{(CN)}_{6}}]}^{4-}}\]is\[+\,2.\] \[F{{e}^{2+}}-1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}},4{{s}^{0}}4p\]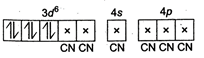 Since \[\text{C}{{\text{N}}^{-}}\]is a strong field ligand, pairing occurs and the hybridisation of \[{{[Fe{{(CN)}_{6}}]}^{4-}}\]is \[{{d}^{2}}s{{p}^{3}}\]and structure is octahedral.
Since \[\text{C}{{\text{N}}^{-}}\]is a strong field ligand, pairing occurs and the hybridisation of \[{{[Fe{{(CN)}_{6}}]}^{4-}}\]is \[{{d}^{2}}s{{p}^{3}}\]and structure is octahedral.
You need to login to perform this action.
You will be redirected in
3 sec
