question_answer 1) If 100 kWh of energy is consumed at 33 V in a copper voltameter, what is the mass of copper liberated? (Take ECE for copper as \[0.33\times {{10}^{-}}^{6}kg/C\])
A)
1 mg
done
clear
B)
1 kg
done
clear
C)
3.6kg
done
clear
D)
3.3kg
done
clear
View Answer play_arrow
question_answer 2) A thin rectangular magnet suspended freely has a period of oscillation of 4 s. If it is broken into two equal halves, the period of oscillation of each half will be
A)
4 s
done
clear
B)
2 s
done
clear
C)
0.5 s
done
clear
D)
0.25 s
done
clear
View Answer play_arrow
question_answer 3) The thermo emf of a thermocouple is given by\[e=2164r-6.2{{t}^{2}},\] the neutral temperature and a temperature of inversion are
A)
349, 174.5
done
clear
B)
174.5, 349
done
clear
C)
349, 698
done
clear
D)
698, 349
done
clear
View Answer play_arrow
question_answer 4) 125 cm of potentiometer wire balances the emf of a cell and 100 cm of the wire is required to balance the poles of a cell which are joined by a 20 resistor. The internal resistance of the cell is
A)
0.250
done
clear
B)
0.500
done
clear
C)
0.750
done
clear
D)
1.250
done
clear
View Answer play_arrow
question_answer 5) A steel wire is 1 m long and has \[1\text{ }m{{m}^{2}}\]area of cross-section. If it takes 200 N to stretch thin wire by 1 mm, how much force will be required to stretch a wire of same material and diameter from its normal length of 10 m to a length of 10.02 m?
A)
1000 N
done
clear
B)
200 N
done
clear
C)
2000 N
done
clear
D)
400 N
done
clear
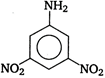
View Answer play_arrow
question_answer 6) A metal sphere cools at the rate of \[3{}^\circ C/min\] when its temperature is \[50{}^\circ C\]. Its rate of cooling when its temperature is \[40{}^\circ C\] and the temperature of surrounding is \[25{}^\circ C\] is
A)
\[1.3{}^\circ C/min\]
done
clear
B)
\[1.4{}^\circ C/min\]
done
clear
C)
\[1.5{}^\circ C/min\]
done
clear
D)
\[1.8{}^\circ C/min\]
done
clear
View Answer play_arrow
question_answer 7) When the rate of emission of radiant heat from a body is less than the rate of absorption of radiant heat from surrounding then the temperature of body will
A)
increase and then decrease
done
clear
B)
remain constant
done
clear
C)
decrease
done
clear
D)
increase
done
clear
View Answer play_arrow
question_answer 8) The limbs of a U-tube are vertical and have internal diameters 0.5 cm and 1 cm respectively. If the tube contains water, what will be the difference in the surface level in the limbs? (Surface tension of water = 72 dyne/cm)
A)
0.2938cm
done
clear
B)
0.8938cm
done
clear
C)
0.6938cm
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 9) A large number of liquid drops each of radius a coalesce to form a single spherical drop of radius b. The energy released in the process is converted into the kinetic energy of the big drop formed, the speed of the big drop is
A)
\[\sqrt{\frac{4T}{\rho }\left( \frac{1}{a}-\frac{1}{b} \right)}\]
done
clear
B)
\[\sqrt{\frac{2T}{\rho }\left( \frac{1}{a}-\frac{1}{b} \right)}\]
done
clear
C)
\[\sqrt{\frac{T}{\rho }\left( \frac{1}{a}-\frac{1}{b} \right)}\]
done
clear
D)
\[\sqrt{\frac{6T}{\rho }\left( \frac{1}{a}-\frac{1}{b} \right)}\]
done
clear
View Answer play_arrow
question_answer 10) The distance between the sun and the earth be r, then the angular momentum of the earth around the sun is proportional to
A)
\[\sqrt{r}\]
done
clear
B)
r3/2
done
clear
C)
r
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 11) The root mean square velocity of the molecules in a sample of helium is \[\frac{5}{7}\]th that of the molecules in a simple of hydrogen. If the temperature of hydrogen sample is \[0{}^\circ C\] then that of helium sample is about
A)
\[100{}^\circ C\]
done
clear
B)
\[273{}^\circ C\]
done
clear
C)
\[173\text{ }K\]
done
clear
D)
\[0{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 12) When a number of thermocouple are joined in series, the thermo emf
A)
is decreased
done
clear
B)
is increased
done
clear
C)
becomes zero
done
clear
D)
remains same
done
clear
View Answer play_arrow
question_answer 13) To cool the car engineers in radiators, water is used because it is having
A)
high value of specific heat
done
clear
B)
high density
done
clear
C)
low surface tension
done
clear
D)
low density
done
clear
View Answer play_arrow
question_answer 14) An SHM is given by y = 5 [sin (3\[\pi \]t) + \[\sqrt{3}\]cos (3\[\pi \]t)] What is the amplitude of the motion of y in metre?
A)
10
done
clear
B)
20
done
clear
C)
1
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 15) When cathode rays are suddenly stopped by metal target such as tungsten, then
A)
X-rays are produced
done
clear
B)
\[\beta -\]rays are produced
done
clear
C)
Neither nor
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 16) Photons of energy 6 eV are incident on a potassium surface of work function 2.1 eV. The stopping potential will be
A)
-8.1V
done
clear
B)
-3.9V
done
clear
C)
-6V
done
clear
D)
-2.1V
done
clear
View Answer play_arrow
question_answer 17) A ray of light is incident on the surface of a medium with the velocity of light at angle of \[45{}^\circ \] and refracted in medium at an angle of \[30{}^\circ \]. What will be the velocity of light in the medium?
A)
\[1.96\times {{10}^{8}}m/s\]
done
clear
B)
\[2.12\times {{10}^{8}}m/s\]
done
clear
C)
\[3.18\times {{10}^{8}}m/s\]
done
clear
D)
\[3.33\times {{10}^{8}}m/s\]
done
clear
View Answer play_arrow
question_answer 18) The type of bonding in a silicon crystal is
A)
van der Waals
done
clear
B)
covalent
done
clear
C)
metallic
done
clear
D)
ionic
done
clear
View Answer play_arrow
question_answer 19) The Rydberg constant for hydrogen is 10967700/m. The shortest and longest wavelength limit in its Lyman series will be respectively
A)
\[911\overset{\text{o}}{\mathop{\text{A}}}\,,\text{ }1215\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[1011\overset{\text{o}}{\mathop{\text{A}}}\,,\text{ }1515\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[711\overset{\text{o}}{\mathop{\text{A}}}\,,\text{ }575\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 20) The quality factor (Q value) of the circuit, when a series resonant circuit contains \[\text{L=}\frac{\text{5}}{\text{ }\!\!\pi\!\!\text{ }}\text{mH,}\,\text{C=}\frac{\text{200}}{\text{ }\!\!\pi\!\!\text{ }}\text{ }\!\!\mu\!\!\text{ F}\]and R= 100 \[\Omega \], will be (source of emf e =200 sin 100 \[\pi \]t is applied to the circuit)
A)
\[5\times {{10}^{-2}}\]
done
clear
B)
\[5\times {{10}^{-5}}\]
done
clear
C)
\[\left( \frac{5}{\pi } \right){{10}^{-5}}\]
done
clear
D)
\[\left( \frac{5}{\pi } \right){{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 21) A ferromagnetic material is placed in an external magnetic field. The magnetic
A)
domains may
done
clear
B)
increase in size
done
clear
C)
decrease in size
done
clear
D)
increase or decrease in size
done
clear
View Answer play_arrow
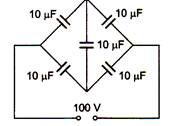
question_answer 22)
Have no relation with the field five capacitors of 10 \[\mu \]F capacity each are connected to a DC potential difference of 100 V as shown in the figure. The equivalent capacitance between the point A and B will be equal to
A)
40 \[\mu F\]
done
clear
B)
20 \[\mu F\]
done
clear
C)
30 \[\mu F\]
done
clear
D)
10 \[\mu F\]
done
clear
View Answer play_arrow
question_answer 23) An electric wire of area of cross-section a and length \[l\] has resistance R. Another wire of same material and same length has cross-sectional area 4a, then the resistance of another wire is
A)
4R
done
clear
B)
\[\frac{R}{4}\]
done
clear
C)
\[\frac{R}{16}\]
done
clear
D)
16R
done
clear
View Answer play_arrow
question_answer 24) The temperature coefficient of resistance of a wire of \[0.00125/{}^\circ C\]. At 300 K, its resistance is 1 \[\Omega \]. The resistance of the wire will be 2\[\Omega \], at following temperature
A)
1127K
done
clear
B)
1128K
done
clear
C)
600 K
done
clear
D)
1400 K
done
clear
View Answer play_arrow
question_answer 25) Two bulbs A and B are connected in parallel. Bulb A will glow more than bulb B. If their resistances are \[{{R}_{A}}\] and \[{{R}_{B}}\] respectively. Then
A)
\[{{R}_{A}}<{{R}_{B}}\]
done
clear
B)
\[{{R}_{A}}={{R}_{B}}\]
done
clear
C)
\[{{R}_{A}}>{{R}_{B}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 26) The torque t acting on a magnet due to a magnetic field B is
A)
\[\tau =B.M\]
done
clear
B)
\[\tau =M\times B\]
done
clear
C)
\[\tau =\frac{B}{M}\]
done
clear
D)
\[\tau =\frac{M}{B}\]
done
clear
View Answer play_arrow
question_answer 27) An ammeter, whose resistance is \[50\Omega \] can read upto 10 mA. In order to increases its range to 1 A. What should be the shunt resistance?
A)
0.5050\[\Omega \]
done
clear
B)
5.050\[\Omega \]
done
clear
C)
50.50 \[\Omega \]
done
clear
D)
505.05\[\Omega \]
done
clear
View Answer play_arrow
question_answer 28) If a particle takes t second less and acquires a velocity of v m/s more in falling through the same distance on two planets where the accelerations due to gravity are 2g and 8g respectively then
A)
v= 4gt
done
clear
B)
v= 5gt
done
clear
C)
v= 2gt
done
clear
D)
v= 16gr
done
clear
View Answer play_arrow
question_answer 29) The energy density of an electromagnetic wave given by \[E=(50N/C)sin(\omega t-kx)\]will be nearly
A)
\[{{10}^{-8}}J{{m}^{-3}}\]
done
clear
B)
\[{{10}^{-7}}J{{m}^{-3}}\]
done
clear
C)
\[{{10}^{-}}^{6}J{{m}^{-3}}\]
done
clear
D)
\[{{10}^{-5}}J{{m}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 30) A calcite crystal is placed over a dot on a piece of paper and rotated. On viewing through calcite one will see
A)
a single dot
done
clear
B)
two stationary dots
done
clear
C)
two rotating dots
done
clear
D)
one rotating about the other
done
clear
View Answer play_arrow
question_answer 31) In the radioactive decay law \[N={{N}_{0}}{{e}^{-}}^{\lambda t}\]the dimensions of \[\lambda \] are
A)
\[\left[ {{\text{M}}^{0}}\text{ }{{\text{L}}^{\text{0}}}\text{ }{{\text{T}}^{\text{0}}} \right]~~~~\]
done
clear
B)
\[\left[ {{\text{M}}^{0}}\text{ }{{\text{L}}^{\text{0}}}\text{ }{{\text{T}}^{-1}} \right]~~~~\]
done
clear
C)
\[\left[ {{\text{M}}^{0}}\text{ }{{\text{L}}^{\text{0}}}\text{ T} \right]~~~~\]
done
clear
D)
\[\left[ \text{M }{{\text{L}}^{\text{0}}}\text{ }{{\text{T}}^{-1}} \right]~~~~\]
done
clear
View Answer play_arrow
question_answer 32) A particle is moving on a circular path with a constant speed v. The magnitude of the change in its velocity after it has described an angle of \[60{}^\circ \] is
A)
\[v\]
done
clear
B)
\[\sqrt{2v}\]
done
clear
C)
\[\sqrt{3v}\]
done
clear
D)
\[\frac{\sqrt{3}}{2}v\]
done
clear
View Answer play_arrow
question_answer 33) A particle moves is X-Y plane under the influence of a force F such that its instantaneous momentum is \[\mathbf{p}=\mathbf{i}2\cos t~+\mathbf{j}2\sin t,\] what is the angle between the force and instantaneous momentum?
A)
\[0{}^\circ \]
done
clear
B)
\[180{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[45{}^\circ \]
done
clear
View Answer play_arrow
question_answer 34) The KE of a body varies directly as the time (t) elapsed. The force acting varies directly as
A)
\[{{t}^{-1}}\]
done
clear
B)
\[{{t}^{-1/2}}\]
done
clear
C)
\[{{t}^{1/2}}\]
done
clear
D)
t
done
clear
View Answer play_arrow
question_answer 35) A parallel plate capacitor is connected across a 2 V battery and charged. The battery is then disconnected and a glass slab is introduced between plates. Which of the following pairs of quantities decrease?
A)
Charge and potential difference
done
clear
B)
Potential difference and energy stored
done
clear
C)
Energy stored and capacitance
done
clear
D)
Capacitance and charge
done
clear
View Answer play_arrow
question_answer 36) A solenoid 600 mm long has 50 turns on it and is wound on an iron rod of 7.5 mm radius. Find the flux through the solenoid when the current in it is 3 A. The relative permeability of iron is 600.
A)
1.66Wb
done
clear
B)
1.66 N Wb
done
clear
C)
1.66 m Wb
done
clear
D)
1.66 \[\mu \] Wb
done
clear
View Answer play_arrow
question_answer 37) A transformer has an efficiency of 80%. It works at 4 kW and 100 V. If secondary voltage is 240 V, the current in primary coil is
A)
0.4 A
done
clear
B)
4 A
done
clear
C)
10 A
done
clear
D)
40 A
done
clear
View Answer play_arrow
question_answer 38) The density for simple cubic lattice is (where A is atomic weight, N is Avogadro number and a is a lattice parameter)
A)
\[\frac{4A}{N{{a}^{3}}}\]
done
clear
B)
\[\frac{2A}{N{{a}^{3}}}\]
done
clear
C)
\[\frac{A}{N{{a}^{3}}}\]
done
clear
D)
\[\frac{A}{N{{a}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 39) A mass of dry air at NTP is compressed to \[\frac{1}{20}\]th of its original volume suddenly. If r = 1.4, the final pressure would be
A)
20atm
done
clear
B)
66.28 atm
done
clear
C)
30 atm
done
clear
D)
150 atm
done
clear
View Answer play_arrow
question_answer 40) A gas at pressure \[6\times {{10}^{s}}N/{{m}^{2}}\]and volume \[1{{m}^{3}}\]expands to \[3\text{ }{{m}^{3}}\] and its pressure falls to\[4\times {{10}^{5}}N/{{m}^{2}}.\] Given, that the indicator diagram is a straight line, work done by the system is
A)
\[6\times {{10}^{5}}J\]
done
clear
B)
\[3\times {{10}^{5}}J\]
done
clear
C)
\[4\times {{10}^{5}}J\]
done
clear
D)
\[10\times {{10}^{5}}J\]
done
clear
View Answer play_arrow
question_answer 41) The distance between two points differing in phase by 60° on a wave having a wave velocity 360 m/s and frequency 500 Hz is
A)
0.72m
done
clear
B)
0.12m
done
clear
C)
0.18m
done
clear
D)
0.36m
done
clear
View Answer play_arrow
question_answer 42) The ionization energy of a hydrogen atom is 13.6 eV. Following Bohrs theory, the energy corresponding to a transition between 3rd and 4th orbit is
A)
3.40 eV
done
clear
B)
1.51 eV
done
clear
C)
0.85 eV
done
clear
D)
0.66 eV
done
clear
View Answer play_arrow
question_answer 43) Neutron decay in free space is given as follows\[_{0}\,n{{\,}^{1}}\,\to {{\,}_{1}}{{H}^{1}}\,+{{\,}_{-}}\,1\,{{e}^{0}}\,+\,[\,]\] Then the parenthesis represents a
A)
neutrino
done
clear
B)
photon
done
clear
C)
antineutrino
done
clear
D)
graviton
done
clear
View Answer play_arrow
question_answer 44) What is the % error in measurement of T of a pendulum, if maximum errors in measurements of (and g are 2% and 4% respectively?
A)
6%
done
clear
B)
3%
done
clear
C)
4%
done
clear
D)
5%
done
clear
View Answer play_arrow
question_answer 45) The masses of neutron and proton are 1.0087 and 1.0073 amu respectively. If the neutrons and protons combine to form helium nucleus of mass 4.0015 amu the binding energy of the helium nucleus will be
A)
28.4 MeV
done
clear
B)
20.8 MeV
done
clear
C)
27.3 MeV
done
clear
D)
14.2 MeV
done
clear
View Answer play_arrow
question_answer 46) The ratio of the energy of a photon of \[~2000\overset{\text{o}}{\mathop{\text{A}}}\,\] wavelength radiation to that of \[~4000\overset{\text{o}}{\mathop{\text{A}}}\,\] radiation is
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{4}\]
done
clear
C)
\[2\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 47) Consider the ground state of an atom \[(Z=24)\]. The numbers of electrons with the azimuthal quantum number \[l=1\] and 2 are respectively.
A)
12 and 4
done
clear
B)
12 and 5
done
clear
C)
16 and 4
done
clear
D)
16 and 5
done
clear
View Answer play_arrow
question_answer 48) The half-life of \[1g\] of radioactive sample is 9 h. The time requires for the original sample to reduce to \[0.2g\]is
A)
\[2.09h\]
done
clear
B)
\[15.6h\]
done
clear
C)
\[20.9h\]
done
clear
D)
\[156h\]
done
clear
View Answer play_arrow
question_answer 49) As the 5-character of hybridisation orbital increases, the bond angle
A)
increases
done
clear
B)
decreases
done
clear
C)
first increases then decreases
done
clear
D)
does not change
done
clear
View Answer play_arrow
question_answer 50) Which of the following halides has maximum melting point?
A)
\[NaF\]
done
clear
B)
\[NaCl\]
done
clear
C)
\[NaBr\]
done
clear
D)
\[NaI\]
done
clear
View Answer play_arrow
question_answer 51) Mark out the incorrect statement about liquid junction potential.
A)
LJP is an additional source of potential difference across the interface of two electrolytes.
done
clear
B)
LJP arises due to different ionic mobility in the different liquids.
done
clear
C)
LJP is essential part of electrolyte concentration cell.
done
clear
D)
Salt bridge promotes liquid junction potential.
done
clear
View Answer play_arrow
question_answer 52) Temperature of one mole of an ideal gas contained in a closed container is increased from \[300K\]to\[301K\]. Pressure-volume work obtained is
A)
\[8.314J\]
done
clear
B)
\[2.494\text{ }kJ\]
done
clear
C)
\[0.8314\text{ }J\]
done
clear
D)
\[83.14\text{ }J\]
done
clear
View Answer play_arrow
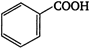
question_answer 53) Aqueous solution of sodium bicarbonate can fairly be useful to distinguish aliphatic carboxylic acid from
A)
done
clear
B)
done
clear
C)
done
clear
D)
\[\underset{COOH}{\overset{COOH}{\mathop{|}}}\,\]
done
clear
View Answer play_arrow
question_answer 54) Leveling bulb is used during experiment to study kinetics of the dissociation of hydrogen peroxide to ensure
A)
uniform pressure difference between the room and the gases in the system
done
clear
B)
pressure within the reaction vessel is same as that in the room
done
clear
C)
same temperature as that of room
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 55) A hydrogen gas electrode has potential of \[-0.118V\]when \[{{H}_{2}}\]as is bubbled at \[298K\] and \[1\text{ }arm\]in \[HCl\] solution. The pH of \[HCl\] solution is
A)
\[2\]
done
clear
B)
\[1\]
done
clear
C)
\[7\]
done
clear
D)
\[2.7\]
done
clear
View Answer play_arrow
question_answer 56) Lassaignes test for the detection of nitrogen fails in
A)
\[N{{H}_{2}}CONH.N{{H}_{2}}.HCl\]
done
clear
B)
\[N{{H}_{2}}N{{H}_{2}}.HCl\]
done
clear
C)
\[N{{H}_{2}}CON{{H}_{2}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}NH.\,N{{H}_{2}}.HCl\]
done
clear
View Answer play_arrow
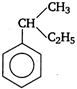
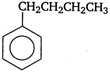
question_answer 57) Among the following, the aromatic compound is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
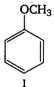
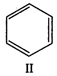
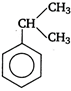
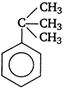
question_answer 58)
Among the following compounds (I - III), the correct order of reaction with electrophilic reagent is
A)
\[II>III>I\]
done
clear
B)
\[III<I<II\]
done
clear
C)
\[I>II>III\]
done
clear
D)
\[I=II>III\]
done
clear
View Answer play_arrow
question_answer 59) The IUPAC name of \[OCH-C{{H}_{2}}-C{{H}_{2}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{2}}-COOH\] is
A)
1-formyl-3-oxopentanoic acid
done
clear
B)
5-formyl-3-oxopentanoic acid
done
clear
C)
3-oxo-5-formylpentanoic acid
done
clear
D)
3-oxo-1-formylpentanoic acid
done
clear
View Answer play_arrow
question_answer 60) Geometrical isomerism is shown by
A)
2-butene
done
clear
B)
2-butyne
done
clear
C)
2-butanol
done
clear
D)
butanal
done
clear
View Answer play_arrow
question_answer 61) One mole of calcium phosphide with excess water gives
A)
one mole of phosphine
done
clear
B)
two moles of phosphoric acid
done
clear
C)
two moles of phosphine
done
clear
D)
one mole of phosphorus pentoxide
done
clear
View Answer play_arrow
question_answer 62) Slow acting nitrogenous fertilizer among the following is
A)
\[CaNCN\]
done
clear
B)
\[N{{H}_{2}}CON{{H}_{2}}\]
done
clear
C)
\[KN{{O}_{3}}\]
done
clear
D)
\[N{{H}_{4}}N{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 63) Which of the following hydroxide is insoluble in water?
A)
\[Ba{{(OH)}_{2}}\]
done
clear
B)
\[Ca{{(OH)}_{2}}\]
done
clear
C)
\[Be{{(OH)}_{2}}\]
done
clear
D)
\[Mg{{(OH)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 64) Which of the following is known as inorganic benzene?
A)
Borazine
done
clear
B)
Phosphonitrilic acid
done
clear
C)
Boron nitride
done
clear
D)
p-dichlorobenzene
done
clear
View Answer play_arrow
question_answer 65) Which of the following attacks on glass?
A)
\[HBr\]
done
clear
B)
\[HCl\]
done
clear
C)
\[HI\]
done
clear
D)
HF
done
clear
View Answer play_arrow
question_answer 66) The number of \[S-S\] bonds in sulphur trioxide is
A)
three
done
clear
B)
two
done
clear
C)
one
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 67) The reaction of chloroform with alcoholic \[KOH\] and p-toluidine forms
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 68) In the conversion of starch to ethyl alcohol, the following enzymes are used
A)
invertase, zymase, pepsin
done
clear
B)
maltase, zymase, yeast
done
clear
C)
diastase, maltase, zymase
done
clear
D)
invertase, diastase, zymase
done
clear
View Answer play_arrow
question_answer 69) Absolute alcohol (100% alcohol) is prepared by distilling rectified spirit over
A)
\[Na\]
done
clear
B)
\[CaC{{l}_{2}}\]
done
clear
C)
\[Mg\]
done
clear
D)
\[Mg{{(O{{C}_{2}}{{H}_{5}})}_{2}}\]
done
clear
View Answer play_arrow
question_answer 70) The reagent used for the preparation of higher ethers from halogenated ethers is
A)
cone. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
sodium alkoxide
done
clear
C)
dry silver oxide
done
clear
D)
Grignard reagent
done
clear
View Answer play_arrow
question_answer 71) One mole of the complex compound \[Co{{(N{{H}_{3}})}_{5}}C{{l}_{2}}\] gives 3 moles of ions on dissolution in water. One mole of the same complex reacts with two moles of \[AgN{{O}_{3}}\] solution to yield two moles of \[AgCl(s)\]. The structure of the complex is
A)
\[[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}].2N{{H}_{3}}\]
done
clear
B)
\[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl.N{{H}_{3}}\]
done
clear
C)
\[[Co{{(N{{H}_{3}})}_{4}}Cl]C{{l}_{2}}.N{{H}_{3}}\]
done
clear
D)
\[[Co{{(N{{H}_{3}})}_{5}}Cl]C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 72) van-Arkel method of purification of metals involves converting the metal to a
A)
volatile stable compound
done
clear
B)
non-volatile stable compound
done
clear
C)
volatile unstable compound
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 73) For the reaction \[A\xrightarrow{{}}B\], the rate expression is \[r=k{{[A]}^{n}}\]. When the concentration of A is doubled, the rate of reaction is quadrupled. The value of n is
A)
\[1\]
done
clear
B)
zero
done
clear
C)
\[3\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 74) On increasing the temperature, rate of reaction increases due to
A)
more number of collisions
done
clear
B)
decrease in mean free path
done
clear
C)
more number of energetic electrons
done
clear
D)
less number of energetic electrons
done
clear
View Answer play_arrow
question_answer 75) \[10\text{ }g\]of a non-volatile solute when dissolved in \[100g\]of benzene raises its boiling point by \[{{1}^{o}}\]. What is the molecular mass of the solute? (\[{{K}_{b}}\] for benzene \[=2.53\,k{{m}^{-1}}\])
A)
\[352\]
done
clear
B)
\[253\]
done
clear
C)
\[275\]
done
clear
D)
\[310\]
done
clear
View Answer play_arrow
question_answer 76) \[0.2mole\]of \[HCl\] and \[0.1\text{ }mole\]of \[CaC{{l}_{2}}\] were dissolved in water to make \[500mL\]of solution. The molarity of \[C{{l}^{-}}\] ions in the solution is
A)
\[0.04\text{ }M\]
done
clear
B)
\[0.8M\]
done
clear
C)
\[0.4M\]
done
clear
D)
\[0.08M\]
done
clear
View Answer play_arrow
question_answer 77) In Ziegler-Natta polymerisation of ethylene, the active species is
A)
\[AlC{{l}_{3}}\]
done
clear
B)
\[TiC{{l}_{4}}\]
done
clear
C)
\[E{{t}_{3}}Al\]
done
clear
D)
\[Ti(III)\]
done
clear
View Answer play_arrow
question_answer 78) Amylopectm is a polymer of
A)
\[\alpha \]-D-glucose
done
clear
B)
\[\alpha \]-D-fructose
done
clear
C)
lactose
done
clear
D)
amylose
done
clear
View Answer play_arrow
question_answer 79) A compound \[CuCl\]has fee structure. Its density is \[3.4\text{ }ge{{m}^{-3}}\]. What is the length of unit cell?
A)
\[4.521\overset{\text{o}}{\mathop{\text{A}}}\,~\]
done
clear
B)
\[5.783\overset{\text{o}}{\mathop{\text{A}}}\,~\]
done
clear
C)
\[5.213\overset{\text{o}}{\mathop{\text{A}}}\,~\]
done
clear
D)
\[4.976\overset{\text{o}}{\mathop{\text{A}}}\,~\]
done
clear
View Answer play_arrow
question_answer 80) \[2moles\]of \[PC{{l}_{5}}\] were heated in a closed vessel of 2L capacity. At equilibrium, 40% of \[PC{{l}_{5}}\] is dissociated into \[PC{{l}_{3}}\] and\[C{{l}_{2}}\]. The value of equilibrium constant is
A)
\[0.266\]
done
clear
B)
\[0.366\]
done
clear
C)
\[2.66\]
done
clear
D)
\[3.66\]
done
clear
View Answer play_arrow
question_answer 81) The solubility product of \[PbC{{l}_{2}}\] at \[{{20}^{o}}C\] is \[1.5\times {{10}^{-4}}\]. Calculate the solubility.
A)
\[3.75\times {{10}^{-4}}\]
done
clear
B)
\[3.34\times {{10}^{-2}}\]
done
clear
C)
\[3.34\times {{10}^{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 82) When solid potassium cyanide is added in water then
A)
pH will increase
done
clear
B)
pH will decrease
done
clear
C)
pH will remain the same
done
clear
D)
electrical conductivity will not change
done
clear
View Answer play_arrow
question_answer 83) Which of the following is true for the reaction \[{{H}_{2}}O(l)\underset{{}}{\overset{{}}{\longleftrightarrow}}{{H}_{2}}O(g)\] at \[{{100}^{o}}C\] and 1 atm?
A)
\[\Delta E=0\]
done
clear
B)
\[\Delta H=0\]
done
clear
C)
\[\Delta H=\Delta E\]
done
clear
D)
\[\Delta H=T.\Delta S\]
done
clear
View Answer play_arrow
question_answer 84) What is when \[1.00\text{ }mole\]of liquid water vaporises at\[{{100}^{o}}C\]? The heat of vaporization. \[\Delta {{H}^{o}}_{vap}\] of water at \[{{100}^{o}}C\] is \[40.66\text{ }kJ\text{ }mo{{l}^{-1}}\].
A)
\[36.73\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
B)
\[40.52\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
C)
\[37.56\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
D)
\[42.05\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 85) The first-four ionisation energy values of an element are\[191\], \[578\] , \[872\]and\[5962\text{ }kcal\]. The number of valence electrons in the element is
A)
\[1\]
done
clear
B)
\[2\]
done
clear
C)
\[3\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 86)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 87) Catalyst \[SnC{{l}_{2}}\] / \[HCl\] is used in
A)
Stephens reduction
done
clear
B)
Cannizaros reaction
done
clear
C)
Clemmensen reduction
done
clear
D)
Rosenmunds reduction
done
clear
View Answer play_arrow
question_answer 88) \[{{C}_{8}}{{H}_{6}}{{O}_{4}}\xrightarrow[{}]{\Delta }X\xrightarrow[{}]{N{{H}_{3}}}Y\] The compound X is
A)
o-xylene
done
clear
B)
phthalic acid
done
clear
C)
phthalic anhydride
done
clear
D)
salicylic acid
done
clear
View Answer play_arrow
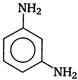
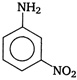
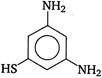
question_answer 89) The major product of the reaction between m-dinitrobenzene and \[N{{H}_{4}}HS\] is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 90) Fog is a colloidal solution of
A)
liquid particles dispersed in gas
done
clear
B)
gaseous particles dispersed in liquid
done
clear
C)
solid particles dispersed in gas
done
clear
D)
solid particles dispersed in liquid butanal
done
clear
View Answer play_arrow
question_answer 91) Algae for the manufacture of iodine is
A)
Diatoms
done
clear
B)
Laminaria
done
clear
C)
Nostoc
done
clear
D)
Polysiphonia
done
clear
View Answer play_arrow
question_answer 92) Powdery mildew is caused by a member of
A)
Basidiomycetes
done
clear
B)
Phycomycetes
done
clear
C)
Ascomycetes
done
clear
D)
Deuteromycetes
done
clear
View Answer play_arrow
question_answer 93) Apophysis is
A)
upper part of moss capsule
done
clear
B)
lower part of seta
done
clear
C)
basal part of sporangium
done
clear
D)
nutritive tissue of sporangium
done
clear
View Answer play_arrow
question_answer 94) Which does not show heterospory?
A)
Pinus
done
clear
B)
Selaginella
done
clear
C)
Dryopteris and moss
done
clear
D)
Sunflower
done
clear
View Answer play_arrow
question_answer 95) Which is called a living fossil?
A)
Cycas
done
clear
B)
Pinus
done
clear
C)
Selaginella
done
clear
D)
Sequoia
done
clear
View Answer play_arrow
question_answer 96) Scutellum of maize is called
A)
coleorhiza
done
clear
B)
cotyledon
done
clear
C)
coleoptile
done
clear
D)
hypocotyls
done
clear
View Answer play_arrow
question_answer 97) In plants like mentha and wild strawberry the stem is
A)
stolon
done
clear
B)
offset
done
clear
C)
runner
done
clear
D)
sucker
done
clear
View Answer play_arrow
question_answer 98) Rootstock is
A)
found in Musa and Alocasia
done
clear
B)
is underground stem
done
clear
C)
Specialized rhizome growing up vertically instead of horizontally
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 99) Passiflora is an example of
A)
cladode
done
clear
B)
thorn
done
clear
C)
stem tendril
done
clear
D)
phylloclade
done
clear
View Answer play_arrow
question_answer 100) Fibrous roots in maize develop from
A)
lower intemodes
done
clear
B)
lower nodes
done
clear
C)
upper nodes
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 101) In which of these plants are all roots?
A)
Wolffia
done
clear
B)
Podostemon
done
clear
C)
Lemna
done
clear
D)
Utricularia
done
clear
View Answer play_arrow
question_answer 102) Root cap is absent in
A)
mesophytes
done
clear
B)
hydrophytes
done
clear
C)
xerophytes
done
clear
D)
lithophytes
done
clear
View Answer play_arrow
question_answer 103) The petiole modified into lamina like structure is found in
A)
Acacia nilotica
done
clear
B)
Acacia Arabica
done
clear
C)
Acacia Australasia
done
clear
D)
Acacia auriculiformis
done
clear
View Answer play_arrow
question_answer 104) Placenianon of Mimosa pudica is
A)
basal
done
clear
B)
marginal
done
clear
C)
axial
done
clear
D)
parietal
done
clear
View Answer play_arrow
question_answer 105) Rhipidium inflorescence in Solanum nigrum is modification of
A)
uniparous cyme
done
clear
B)
multiparouschyme
done
clear
C)
scorpioid
done
clear
D)
capitulum
done
clear
View Answer play_arrow
question_answer 106) The glumes are modified
A)
petals
done
clear
B)
sepals
done
clear
C)
tepals
done
clear
D)
bracts
done
clear
View Answer play_arrow
question_answer 107) Catkin inflorescence is characteristic of
A)
Morus
done
clear
B)
Tritium
done
clear
C)
Focus
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 108) An aggregate of simple fruit borne by single flower is
A)
berry
done
clear
B)
cypsela
done
clear
C)
etaerio
done
clear
D)
caryopsis
done
clear
View Answer play_arrow
question_answer 109) Perisperm is the
A)
remains of nucellus
done
clear
B)
outer part of embryo sac
done
clear
C)
degenerated synergid
done
clear
D)
degenerated secondary nucleus
done
clear
View Answer play_arrow
question_answer 110) Winged fruits favouring anemochory occur in
A)
cypsela
done
clear
B)
samara
done
clear
C)
regma
done
clear
D)
siliqua
done
clear
View Answer play_arrow
question_answer 111) Syngenesious condition is found in family
A)
Liliaceae
done
clear
B)
Malvaceae
done
clear
C)
Compositae
done
clear
D)
Cruciferae
done
clear
View Answer play_arrow
question_answer 112) The tissue in the roots to absorb water and minerals is
A)
epidermal appendages
done
clear
B)
epidermal extensions
done
clear
C)
hypodermis
done
clear
D)
endodermis
done
clear
View Answer play_arrow
question_answer 113) Triradiate vascular bundles can be traced in
A)
monocot roots
done
clear
B)
dicot root
done
clear
C)
monocots
done
clear
D)
dicot stem
done
clear
View Answer play_arrow
question_answer 114) In which of the following are inulin crystals found?
A)
Wheat root
done
clear
B)
Mango root
done
clear
C)
Dahlia root
done
clear
D)
Sugarcane root
done
clear
View Answer play_arrow
question_answer 115) Two to six xylem and phloem patches are present in
A)
dicot root
done
clear
B)
monocot root
done
clear
C)
root hair
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 116) Diffuse porous woods are characteristic of plants growing in
A)
alpine region
done
clear
B)
cold winter region
done
clear
C)
temperate climate
done
clear
D)
tropics
done
clear
View Answer play_arrow
question_answer 117) The water vapour of a plant sample or water solution is measured by
A)
auxanometer
done
clear
B)
psychrometer
done
clear
C)
osmometer
done
clear
D)
photometer
done
clear
View Answer play_arrow
question_answer 118) When the temperature of soil is 0°C then the water absorption
A)
increases
done
clear
B)
decreases
done
clear
C)
not affected
done
clear
D)
soil will lose capillary \[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 119) Endosmosis of water occurs when in comparison with the outer solution the water potential of cell sap is
A)
higher
done
clear
B)
lower
done
clear
C)
equal
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 120) Excess of one of them act as antitranspirant
A)
\[{{H}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 121) Which of the following tree will die first?
A)
Deciduous
done
clear
B)
Pruned
done
clear
C)
Girdled
done
clear
D)
Annual
done
clear
View Answer play_arrow
question_answer 122) Little leaf disease develop due to deficiency of
A)
sodium
done
clear
B)
copper
done
clear
C)
calcium
done
clear
D)
zinc
done
clear
View Answer play_arrow
question_answer 123) For chlorophyll formation most important are
A)
iron and Ca
done
clear
B)
iron and Mg
done
clear
C)
Mg and Ca
done
clear
D)
iron and Na
done
clear
View Answer play_arrow
question_answer 124) Which fractions of the visible spectrum of solar radiations are primarily absorbed by carotenoids of the higher plants?
A)
Blue and green
done
clear
B)
Green and red
done
clear
C)
Red and violet
done
clear
D)
Violet and blue
done
clear
View Answer play_arrow
question_answer 125) What is common in NAD, ATP and FMN?
A)
Zn
done
clear
B)
P
done
clear
C)
Ca
done
clear
D)
Mg
done
clear
View Answer play_arrow
question_answer 126) Secondary dormancy is due to the absence of
A)
suitable plant
done
clear
B)
hormones
done
clear
C)
favourable conditions
done
clear
D)
unfavourable conditions
done
clear
View Answer play_arrow
question_answer 127) Which one has the largest gametophyte?
A)
Oryza
done
clear
B)
Funaria
done
clear
C)
Selaginella
done
clear
D)
Pinus
done
clear
View Answer play_arrow
question_answer 128) Exine of pollen grain is made up of
A)
sporopollenin
done
clear
B)
pectocellulose
done
clear
C)
lingo-cellulose
done
clear
D)
pollenkit
done
clear
View Answer play_arrow
question_answer 129) The pollination is ......... in eel grass
A)
hydrophilous
done
clear
B)
zoophilous
done
clear
C)
entomophilous
done
clear
D)
anemophilous
done
clear
View Answer play_arrow
question_answer 130) The parthenocarpy is not advantageous in
A)
mango
done
clear
B)
coconut
done
clear
C)
pomegranate
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 131) A plant hormone used for inducing morphogenesis in tissue culture is
A)
ABA
done
clear
B)
ethylene
done
clear
C)
gibberellin
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 132) Phytoplanktons in the hydrosere constitute
A)
member of climax community
done
clear
B)
pioneer community
done
clear
C)
intermediate seral members
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 133) Deme evergreen vegetation of broad sclerophyllous leaves and shrubs with fire resistant resisnous plants is known as
A)
chaparral vegetation
done
clear
B)
savannah vegetation
done
clear
C)
steppe grassland
done
clear
D)
tundra vegetation
done
clear
View Answer play_arrow
question_answer 134) The major chunk of nitrogen in nature is fixed by
A)
lightning
done
clear
B)
symbiotic bacteria
done
clear
C)
denitrifying bacteria
done
clear
D)
chemical industries
done
clear
View Answer play_arrow
question_answer 135) A plant which is cultivated by tissue culture technique is
A)
Citrus
done
clear
B)
apple
done
clear
C)
pear
done
clear
D)
guava
done
clear
View Answer play_arrow
question_answer 136) Malaria fever conincides with liberation of
A)
cryptomerozoites
done
clear
B)
metacryptomerozoites
done
clear
C)
merozoites
done
clear
D)
trophozoites
done
clear
View Answer play_arrow
question_answer 137) Bath sponges are formed from
A)
Euplectella
done
clear
B)
dried spongin fibre skeleton
done
clear
C)
skeleton of Spongilla
done
clear
D)
dry Leucosolenia
done
clear
View Answer play_arrow
question_answer 138) Corals are
A)
most rich fauna
done
clear
B)
most poor fauna
done
clear
C)
also found in fresh water
done
clear
D)
free living ctenophorans
done
clear
View Answer play_arrow
question_answer 139) In Planaria, the structure ocelli is
A)
excretory organ
done
clear
B)
light sensitive organ
done
clear
C)
vestigial organ
done
clear
D)
chemoreceptor organ
done
clear
View Answer play_arrow
question_answer 140) In Enterobius vermicularis, the method of transmission is
A)
contamination
done
clear
B)
congenital
done
clear
C)
direct
done
clear
D)
by vector
done
clear
View Answer play_arrow
question_answer 141) Which of the following is the marine leech?
A)
Sipunculus
done
clear
B)
Pontobdella
done
clear
C)
Hirudo
done
clear
D)
Polygordices
done
clear
View Answer play_arrow
question_answer 142) Amphineuran mollusc is
A)
Chiton
done
clear
B)
Nautilus
done
clear
C)
Dentalium
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 143) Proboscis of butterfly is formed from
A)
paraglossae
done
clear
B)
labrum
done
clear
C)
maxillae
done
clear
D)
mandible
done
clear
View Answer play_arrow
question_answer 144) The biological name of feather star is
A)
Ophiothrix
done
clear
B)
Antedon
done
clear
C)
Petaceros
done
clear
D)
Clypeaster
done
clear
View Answer play_arrow
question_answer 145) Excretory organ of Balanoglossus is
A)
Malpighian tubules
done
clear
B)
flame cells
done
clear
C)
nephridia
done
clear
D)
proboscis gland
done
clear
View Answer play_arrow
question_answer 146) Which of the following is commonly called flat fish?
A)
Synaptura
done
clear
B)
Anabas
done
clear
C)
Etroplus
done
clear
D)
Anguilla
done
clear
View Answer play_arrow
question_answer 147) Axoloti is the larva of
A)
Ambystoma
done
clear
B)
Silkworm
done
clear
C)
Roundworm
done
clear
D)
Amphioxus
done
clear
View Answer play_arrow
question_answer 148) Which one of these does not belong to the order Galliformes?
A)
Jungle fowl
done
clear
B)
peacock
done
clear
C)
Phesant
done
clear
D)
Green parrot
done
clear
View Answer play_arrow
question_answer 149) Hairless mammals are
A)
rodents
done
clear
B)
chiropterans
done
clear
C)
primates
done
clear
D)
cetaceans
done
clear
View Answer play_arrow
question_answer 150) Respiratory mesosomes are also known as
A)
mitochondria of bacteria
done
clear
B)
chondrioids
done
clear
C)
store house of respiratory enzyme
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 151) The secretion of macromolecules by fusing a transport vesicle to the plasma membrane is
A)
pinocytosis
done
clear
B)
phagocytosis
done
clear
C)
endocytosis
done
clear
D)
exocytosis
done
clear
View Answer play_arrow
question_answer 152) ATP molecule is
A)
nucleosome
done
clear
B)
nucleoside
done
clear
C)
nucleotide
done
clear
D)
deoxyribose sugar
done
clear
View Answer play_arrow
question_answer 153) Nickel contributes to the formation of one of the following
A)
urease
done
clear
B)
dehydrogenase
done
clear
C)
rubisco protein
done
clear
D)
nitrate reductase
done
clear
View Answer play_arrow
question_answer 154) The prophase-I of the meiotic division has sub-stages.
A)
two
done
clear
B)
three
done
clear
C)
five
done
clear
D)
six
done
clear
View Answer play_arrow
question_answer 155) A submetacentric chromosome has
A)
only one arm
done
clear
B)
V shaped arms
done
clear
C)
L shaped arm
done
clear
D)
Very unequal arms
done
clear
View Answer play_arrow
question_answer 156) Unwinding of DNA is done by
A)
topoisomerase
done
clear
B)
exonuclease
done
clear
C)
helicase
done
clear
D)
ligase
done
clear
View Answer play_arrow
question_answer 157) Transitional epithelium is found in
A)
lungs
done
clear
B)
liver
done
clear
C)
urinary bladder
done
clear
D)
stomach
done
clear
View Answer play_arrow
question_answer 158) Muscles can survive anoxic episodes because they receive ATP in absence of oxygen due to one of the following cycles
A)
glycolysis
done
clear
B)
ETS
done
clear
C)
Krebs cycle
done
clear
D)
HMP pathway
done
clear
View Answer play_arrow
question_answer 159) Epidermis is specialized for
A)
respiration
done
clear
B)
absorption
done
clear
C)
protection
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 160) Ileo-caecal valve in rabbit is present in
A)
duodenum
done
clear
B)
pyloric stomach
done
clear
C)
sacculus rotundus
done
clear
D)
rectum
done
clear
View Answer play_arrow
question_answer 161) Book lungs are respiratory organs of
A)
Mollusca
done
clear
B)
Mammals
done
clear
C)
Arachnida
done
clear
D)
Earthworm
done
clear
View Answer play_arrow
question_answer 162) The movement of neurophils to site of inflammation is due to
A)
release of hormone
done
clear
B)
chemotaxis
done
clear
C)
activity of antigens
done
clear
D)
activation of antigens
done
clear
View Answer play_arrow
question_answer 163) P wave in ECG occurs before the
A)
onset of ventricular ejection
done
clear
B)
end of arterial contraction
done
clear
C)
beginning of atrial contraction
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 164) Kidney stones are crystals of
A)
sodium chloride
done
clear
B)
silica
done
clear
C)
calcium oxalate
done
clear
D)
potassium chloride
done
clear
View Answer play_arrow
question_answer 165) Nissi granules could be seen in
A)
bone cells
done
clear
B)
gland cells
done
clear
C)
myofibrils
done
clear
D)
neurons
done
clear
View Answer play_arrow
question_answer 166) Suspensory ligaments are found in
A)
brain
done
clear
B)
eye
done
clear
C)
ear
done
clear
D)
kidney
done
clear
View Answer play_arrow
question_answer 167) FSH is
A)
glycoprotein
done
clear
B)
catecholamine
done
clear
C)
polypeptide
done
clear
D)
steroid
done
clear
View Answer play_arrow
question_answer 168) The mammalian corpus luteum produces
A)
oestrogen
done
clear
B)
progesterone
done
clear
C)
leuteotropic hormone
done
clear
D)
luteinizing hormone
done
clear
View Answer play_arrow
question_answer 169) Spiral cleavage is found in
A)
nemertines
done
clear
B)
annelids
done
clear
C)
Mollusca
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 170) In animals, chalones are substances responsible for
A)
regeneration
done
clear
B)
ageing
done
clear
C)
parthenogenesis
done
clear
D)
development
done
clear
View Answer play_arrow
question_answer 171) If the age pyramid of a population is bell-shaped. It will show
A)
high % of old
done
clear
B)
high % of children
done
clear
C)
low % of children
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 172) In Bhopal gas tragedy, the gas released was
A)
methyl isocyanate
done
clear
B)
ethyl isocyanate
done
clear
C)
sodium isocyanate
done
clear
D)
potassium isocyanate
done
clear
View Answer play_arrow
question_answer 173) The most important component of the oral contraceptive pills is
A)
progesterone
done
clear
B)
growth hormone
done
clear
C)
thyroxin
done
clear
D)
luteinizing hormone
done
clear
View Answer play_arrow
question_answer 174) The average cup of tea contains ......... mg of caffeine
A)
40
done
clear
B)
10
done
clear
C)
60
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 175) Osteoporosis can result by long use of
A)
antibiotics
done
clear
B)
stimulants
done
clear
C)
steroids
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 176) Ranikhet disease is to
A)
poultry
done
clear
B)
fish
done
clear
C)
pigs
done
clear
D)
honeybee
done
clear
View Answer play_arrow
question_answer 177) The rejection of organ transplanting in humans is prevented by using
A)
aspirin
done
clear
B)
cyclosporine
done
clear
C)
thrombin
done
clear
D)
disprin
done
clear
View Answer play_arrow
question_answer 178) Which of the following in not water borne disease?
A)
Asthma
done
clear
B)
Cholera
done
clear
C)
Amoebiasis
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 179) Which one is a vital stain?
A)
methylene blue
done
clear
B)
janus green
done
clear
C)
neutral red
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 180) Which of these institute prepares antivenins?
A)
CDRI, Lucknow
done
clear
B)
CIT, Lucknow
done
clear
C)
Hoffkin, Bombay
done
clear
D)
CIV, Pune
done
clear
View Answer play_arrow