A) It has planar structure
B) It has one coordinate bond
C) It has three resonating structures
D) Hydrolysis of \[CO_{3}^{2-}\] ion gives basic solution
Correct Answer: B
Solution :
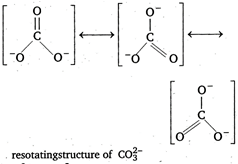 \[CO_{3}^{2-}\] has \[s{{p}^{2}}\] hybridisation ie, planar structure
\[CO_{3}^{2-}\] has \[s{{p}^{2}}\] hybridisation ie, planar structure
You need to login to perform this action.
You will be redirected in
3 sec
