question_answer 1) A rod of length L and mass m is kept vertically on the ground. Its potential energy is
A)
\[mgL\]
done
clear
B)
\[mg\frac{L}{2}\]
done
clear
C)
\[mg\frac{L}{3}\]
done
clear
D)
\[mg\frac{L}{4}\]
done
clear
View Answer play_arrow
question_answer 2) A satellite is put in an orbit at height \[h=R\]. What is change in potential energy?
A)
\[mgR\]
done
clear
B)
\[\frac{mgR}{2}\]
done
clear
C)
\[2mgR\]
done
clear
D)
\[\frac{3}{2}mgR\]
done
clear
View Answer play_arrow
question_answer 3) A body is projected at an angle \[{{45}^{o}}\] with horizontal. If escape velocity when projected vertically up is 11.2 km/s, what is its new escape velocity when projected at the given angle?
A)
\[\frac{11.2}{\sqrt{2}}km/s\]
done
clear
B)
\[11.2\sqrt{2}km/s\]
done
clear
C)
\[11.2\,km/s\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 4) If total energy of satellite is E, what is its potential energy?
A)
\[2E\]
done
clear
B)
\[-2E\]
done
clear
C)
\[E\]
done
clear
D)
\[-E\]
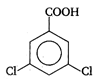
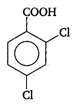
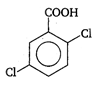
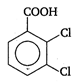
done
clear
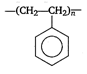
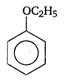
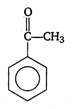
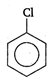
View Answer play_arrow
question_answer 5) If radius of earth is reduced
A)
tide duration is reduced
done
clear
B)
earth rotates slower
done
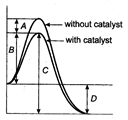
clear
C)
time period of earth decreases
done
clear
D)
duration of day increases
done
clear
View Answer play_arrow
question_answer 6) Zener diode acts as
A)
voltage regulator in reverse biasing
done
clear
B)
voltage regulator in forward biasing
done
clear
C)
current regulator in reverse biasing
done
clear
D)
current regualtor in forward biasing
done
clear
View Answer play_arrow
question_answer 7) What is \[\frac{e}{m}\] ratio of electron?
A)
\[2.76\times {{10}^{10}}\]
done
clear
B)
\[2.76\times {{10}^{11}}\]
done
clear
C)
\[1.76\times {{10}^{10}}\]
done
clear
D)
\[1.76\times {{10}^{11}}\]
done
clear
View Answer play_arrow
question_answer 8) What is charge on 90 kg of electrons?
A)
\[1.58\times {{10}^{13}}\]
done
clear
B)
\[2.23\times {{10}^{12}}\]
done
clear
C)
\[2.53\times {{10}^{12}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 9) At what temperature iron becomes paramagnetic?
A)
\[{{200}^{o}}C\]
done
clear
B)
\[{{400}^{o}}C\]
done
clear
C)
\[{{600}^{o}}C\]
done
clear
D)
\[{{800}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 10) Sparkling of diamond is because of
A)
total internal reflection
done
clear
B)
refraction
done
clear
C)
diffraction
done
clear
D)
scattering
done
clear
View Answer play_arrow
question_answer 11) Pressure variation in a mechanical wave depends upon as
A)
\[\propto \] intensity
done
clear
B)
independent of intensity
done
clear
C)
\[\propto \frac{\text{1}}{\text{intensity}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 12) Earths magnetic field is
A)
\[{{10}^{-4}}T\]
done
clear
B)
\[{{10}^{-5}}T\]
done
clear
C)
\[{{10}^{-6}}T\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 13) If charge and distance between two charges are reduced to half. Force between them
A)
remains same
done
clear
B)
increases four times
done
clear
C)
reduce four times
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 14) Consider a vehicle going on a horizontal road towards east. Neglect any force by the air. The frictional force on the vehicle by the road
A)
is zero if the vehicle is moving with a uniform velocity
done
clear
B)
is towards east if the vehicle is accelerating
done
clear
C)
must be towards east
done
clear
D)
must be towards west
done
clear
View Answer play_arrow
question_answer 15) Masses m and M on pulley move 0.6 m in 4 s. What is ratio of \[\frac{m}{M}\]?
A)
\[\frac{55}{57}\]
done
clear
B)
\[\frac{113}{117}\]
done
clear
C)
\[\frac{57}{55}\]
done
clear
D)
\[\frac{397}{403}\]
done
clear
View Answer play_arrow
question_answer 16) Three rods of equal length of thermal conductivity K, 2K and 3K are symmetrically joined. If temperature of ends are \[{{0}^{o}}C,\,{{50}^{o}}C\]and \[{{100}^{o}}C\]respectively, what is the temperature of the junction?
A)
\[20{{\,}^{o}}C\]
done
clear
B)
\[\frac{100}{3}{}^{o}C\]
done
clear
C)
\[\frac{200}{3}{}^{o}C\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 17) Frequency of cyclotron does not depend upon
A)
charge
done
clear
B)
mass
done
clear
C)
Velocity
done
clear
D)
\[\frac{q}{m}\]
done
clear
View Answer play_arrow
question_answer 18) Number of beats between A and B is 5 and between B and C are 3. Beat frequency between A and C may be
A)
1
done
clear
B)
2
done
clear
C)
8
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 19) A gas at one atmosphere and having volum 100mL is mixed with another gas of equal moles at 0.5atm and having volume 50mL in flask of one litre. What is their final pressure?
A)
0.5atm
done
clear
B)
1atm
done
clear
C)
0.75atm
done
clear
D)
0.125atm
done
clear
View Answer play_arrow
question_answer 20) A body cools from \[{{80}^{o}}C\] to \[{{64}^{o}}C\] in 5 min and same body cools from \[{{80}^{o}}C\] to \[{{52}^{o}}C\] in 10 min, what is the temperature of the surrounding?
A)
\[{{24}^{o}}C\]
done
clear
B)
\[{{28}^{o}}C\]
done
clear
C)
\[{{22}^{o}}C\]
done
clear
D)
\[{{25}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 21) Sky waves are reflected by
A)
statosphere
done
clear
B)
mesosphere
done
clear
C)
ionosphere
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 22) What is size of gold nuclei?
A)
\[3{{R}_{0}}\]
done
clear
B)
\[4{{R}_{0}}\]
done
clear
C)
\[5{{R}_{0}}\]
done
clear
D)
\[5.8{{R}_{0}}\]
done
clear
View Answer play_arrow
question_answer 23) In L-C-R resonant circuit what is the phase angle \[\phi \]?
A)
\[{{90}^{o}}\]
done
clear
B)
\[{{180}^{o}}\]
done
clear
C)
\[{{0}^{o}}\]
done
clear
D)
\[{{60}^{o}}\]
done
clear
View Answer play_arrow
question_answer 24) In R-C circuit \[\omega =100\,\,rad/s,\text{ }R=100\,\,\Omega \],\[\text{C}=20\,\,\mu F\] what is impedence?
A)
\[510\,\,\Omega \]
done
clear
B)
\[200\,\,\Omega \]
done
clear
C)
\[250\,\,\Omega \]
done
clear
D)
\[300\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 25) A body is moving forward and backward Change in frequency observed by the body of a source is 2%. What is velocity of the body? (speed of sound is 300 m/s)
A)
6 m/s
done
clear
B)
2 m/s
done
clear
C)
2.5 m/s
done
clear
D)
3 m/s
done
clear
View Answer play_arrow
question_answer 26) A body is projected horizontally with velocity 196 m/s from height 400 m. What is the time to reach the ground?
A)
5 s
done
clear
B)
9 s
done
clear
C)
15 s
done
clear
D)
20 s
done
clear
View Answer play_arrow
question_answer 27) Two spheres of unequal mass but same radius are released on inclined plane. They rolls down with slipping. Which one will reach the ground first?
A)
Lighter sphere
done
clear
B)
Heavier sphere
done
clear
C)
Both will reach at the same time
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 28) A cylinder is rolling down a inclined plane of inclination \[{{60}^{o}}\]. What is its acceleration?
A)
\[g/\sqrt{3}\]
done
clear
B)
\[g/\sqrt{3}\]
done
clear
C)
\[\sqrt{\frac{2g}{3}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 29) Lead is
A)
diamagnetic
done
clear
B)
paramagnetic
done
clear
C)
ferromagnetic
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 30) The magnetic moment of atomic neon is
A)
zero
done
clear
B)
\[\mu B/2\]
done
clear
C)
\[\mu B\]
done
clear
D)
\[3\mu B/2\]
done
clear
View Answer play_arrow
question_answer 31) In a potentiometer, the null point is received at 7th wire. If now we have to change the null point at the 9th wire, what should we do?
A)
Attach resistance in series with battery
done
clear
B)
Increse resistance in main circuit
done
clear
C)
Decrease resistance in main circuit
done
clear
D)
Decrease applied emf
done
clear
View Answer play_arrow
question_answer 32) Water falls from height 100 m. Temperature of water
A)
increases
done
clear
B)
decreases
done
clear
C)
remains same
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 33) Two identical concentric rings each of mass m and radius R are placed perpendicularly. What is the moment of inertia about axis of one of the rings?
A)
\[\frac{3}{2}M{{R}^{2}}\]
done
clear
B)
\[2M{{R}^{2}}\]
done
clear
C)
\[3M{{R}^{2}}\]
done
clear
D)
\[\frac{1}{4}M{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 34) A motor of power 60 W draws current 5 A from a source of 15 V. What is loss of power?
A)
SOW
done
clear
B)
25 W
done
clear
C)
20 W
done
clear
D)
15 W
done
clear
View Answer play_arrow
question_answer 35) An electron and proton are placed at distance 4.3 nm. What is dipole moment (C-m)?
A)
\[3.44\times {{10}^{-28}}\]
done
clear
B)
\[2\times {{10}^{-28}}\]
done
clear
C)
\[6.88\times {{10}^{-28}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 36)
A)
6A
done
clear
B)
5A
done
clear
C)
7 A
done
clear
D)
8 A
done
clear
View Answer play_arrow
question_answer 37) Police is chasing the thief 50 m ahead. In 10 s distance between them reduces by 6 m. What is distance between them in 25 s?
A)
10m
done
clear
B)
25m
done
clear
C)
35m
done
clear
D)
20m
done
clear
View Answer play_arrow
question_answer 38) A body of mass 10 kg initially at rest acquires velocity 10 m/s. What is the work done?
A)
-500 J
done
clear
B)
500 J
done
clear
C)
50 J
done
clear
D)
-50 J
done
clear
View Answer play_arrow
question_answer 39) A projectile is projected at an angle \[{{60}^{o}}\] with horizontal with speed 10 m/s. The minimum radius of curvature of trajectory described by die projectile is
A)
2.25 m
done
clear
B)
2 m
done
clear
C)
10 m
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 40) What is nature of light and sound waves?
A)
Light is transverse and sound is longitudinal
done
clear
B)
Light is longitudinal and sound is transverse
done
clear
C)
Both are transverse
done
clear
D)
Both are longitudinal
done
clear
View Answer play_arrow
question_answer 41) If the colour code of carbon resistor is as follows, then give the value of its resistance in\[\text{k}\Omega \]. Colour of I strip - yellow Colour of II strip - blue Colour of III strip - orange Colour of IV strip - gold
A)
\[46\pm 5%\]
done
clear
B)
\[0.46\pm 5%\]
done
clear
C)
\[46\pm 10%\]
done
clear
D)
\[0.46\pm 10%\]
done
clear
View Answer play_arrow
question_answer 42) Self-inductance of solenoid is proportional to
A)
\[\frac{NA}{l}\]
done
clear
B)
\[\frac{N{{A}^{2}}}{l}\]
done
clear
C)
\[\frac{A}{l}\]
done
clear
D)
\[\frac{{{N}^{2}}A}{l}\]
done
clear
View Answer play_arrow
question_answer 43) A body is rolling down an inclined plane. If KE of rotation is 40% of KE in translatory state, then the body is a
A)
ring
done
clear
B)
cylinder
done
clear
C)
hollow ball
done
clear
D)
solid ball
done
clear
View Answer play_arrow
question_answer 44) Consider the following equation of Bernoullis theorem \[p+\frac{1}{2}\rho {{V}^{2}}+\rho gh=k\] (Constant) The dimensions of k/p are same as that of which of the following?
A)
Thrus
done
clear
B)
Pressure
done
clear
C)
Angle
done
clear
D)
Viscosity
done
clear
View Answer play_arrow
question_answer 45) Resistance of tungsten wire at \[{{150}^{o}}C\] is \[133\Omega .\]. Its resistance temperature coefficient is \[{{0045}^{o}}/C\]. The resistance of the wire at \[500{}^\circ C\] will be
A)
\[180\,\Omega \]
done
clear
B)
\[225\,\Omega \]
done
clear
C)
\[258\,\Omega \]
done
clear
D)
\[317\,\Omega \]
done
clear
View Answer play_arrow
question_answer 46) In a radioactive decay process, the negatively charged emitted B-particles are
A)
the electrons present inside the nucleus
done
clear
B)
the electrons produced as a result of the decay of neutrons inside the nucleus
done
clear
C)
the electrons produced as a result of collisions between atoms
done
clear
D)
the electrons orbiting around the nucleus
done
clear
View Answer play_arrow
question_answer 47) If an electron jumps from 1st orbit to 3rd orbit, then it will
A)
absorb energy
done
clear
B)
release energy
done
clear
C)
no gain of energy
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 48) Activity of radioactive element decreased to one-third of original activity \[{{R}_{o}}\] in 9 yr. After further 9 yr, its activity will be
A)
\[{{R}_{o}}\]
done
clear
B)
\[\frac{2}{3}{{R}_{o}}\]
done
clear
C)
\[\frac{{{R}_{o}}}{9}\]
done
clear
D)
\[\frac{{{R}_{o}}}{6}\]
done
clear
View Answer play_arrow
question_answer 49) Which of the following four fundamental forces has shortest range?
A)
Nuclear
done
clear
B)
Gravitational
done
clear
C)
Electromagnetic
done
clear
D)
Weak force
done
clear
View Answer play_arrow
question_answer 50) The angle of minimum deviation for a prism is \[{{40}^{o}}\] and the angle of the prism is \[{{60}^{o}}\]. The angle of incidence in this position will be
A)
\[{{30}^{o}}\]
done
clear
B)
\[{{60}^{o}}\]
done
clear
C)
\[{{50}^{o}}\]
done
clear
D)
\[{{100}^{o}}\]
done
clear
View Answer play_arrow
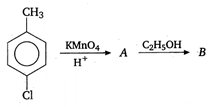
question_answer 51)
Identify B in the following reaction
A)
done
clear
B)
done
clear
C)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 52) Which of the following is chiral?
A)
\[Cl-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
C)
\[Cl-CH=CH-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
D)
\[H-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,=C=CH-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 53) In the given compound which of the following hydrogens is most acidic? \[\underset{(a)}{\mathop{C{{H}_{3}}}}\,-\underset{(b)}{\mathop{C}}\,{{H}_{2}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-\underset{(C)}{\mathop{C{{H}_{3}}}}\,\]
A)
Only [a]
done
clear
B)
Only [b]
done
clear
C)
Only [c]
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 54) The following solids are formed by X, Q and Z.\[X{{Y}_{2}},\,\,{{X}_{2}}Z,\,\,QZ\]. Then the formula of the compound formed by Q and Y is
A)
QY
done
clear
B)
\[{{Q}_{2}}{{Y}_{3}}\]
done
clear
C)
\[Q{{Y}_{4}}\]
done
clear
D)
\[Q{{Y}_{3}}\]
done
clear
View Answer play_arrow
question_answer 55) In the following complex compound\[{{[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]}^{+}}\], Co and \[Cl\] are collinear. Which of the following structures is possible?
A)
linkage
done
clear
B)
trans
done
clear
C)
ci5
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 56)
A)
done
clear
B)
done
clear
C)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 57) Which of the following is false for tetrahedral complexes?
A)
Low spin
done
clear
B)
High spin
done
clear
C)
d-d transition
done
clear
D)
Coloured
done
clear
View Answer play_arrow
question_answer 58) In the following reaction \[NH_{4}^{+}+BiN\xrightarrow{{}}N{{H}_{3}}+B{{i}^{3+}}\] \[NH_{4}^{+}\] is acting as an
A)
oxidising agent
done
clear
B)
acid
done
clear
C)
base
done
clear
D)
catalyst
done
clear
View Answer play_arrow
question_answer 59) Strongest Lewis acid among the following is
A)
\[N{{F}_{3}}\]
done
clear
B)
\[PC{{l}_{3}}\]
done
clear
C)
\[SnC{{l}_{4}}\]
done
clear
D)
\[PbC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 60) \[NaH\], when dissolved in water, produces
A)
acidic medium
done
clear
B)
basic medium
done
clear
C)
neutral medium
done
clear
D)
Cannot be predicted
done
clear
View Answer play_arrow
question_answer 61) In a closed packed structure
A)
tetrahedral voids are bigger than octahedral
done
clear
B)
tetrahedral voids are smaller than octahedral
done
clear
C)
tetrahedral voids are equal in size as octahedral
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 62) An element with configuration\[1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}}3{{p}^{5}}\] will form a compound of highest ionic character with the element having configuration
A)
\[1{{s}^{2}},2{{s}^{2}},2{{p}^{6}}\]
done
clear
B)
\[[Ar]\,4{{s}^{1}},3{{d}^{10}}\]
done
clear
C)
\[[Ar]\,4{{s}^{1}}\]
done
clear
D)
\[1{{s}^{2}},\,2{{s}^{1}}\]
done
clear
View Answer play_arrow
question_answer 63) \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}COOH\xrightarrow{{}}\] \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}-\overset{\begin{smallmatrix} N{{H}_{2}} \\ | \end{smallmatrix}}{\mathop{C}}\,H-COOH\] the reagents used in the conversion are
A)
(i) \[PB{{r}_{3}}/\](ii) \[N{{H}_{3}}\]
done
clear
B)
(i) red P, \[B{{r}_{2}}/\](ii) \[N{{H}_{3}}\] (excess)
done
clear
C)
(i) \[PB{{r}_{3}},NaCN/\] (ii) \[LiAl{{H}_{4}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 64) Empirical formula of compound having molar mass 58 is \[{{C}_{2}}{{H}_{5}}\]. Number of structural isomers possible, are
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
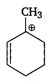
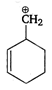
question_answer 65) Most stable carbocation among the following is
A)
done
clear
B)
done
clear
C)
done
clear
D)
All are equally
done
clear
View Answer play_arrow
question_answer 66) Which of the following has highest ionization energy?
A)
\[{{K}^{+}}\]
done
clear
B)
\[C{{l}^{-}}\]
done
clear
C)
Ar
done
clear
D)
\[C{{s}^{+}}\]
done
clear
View Answer play_arrow
question_answer 67)
A)
electrophilic substitution
done
clear
B)
nucleophilic substitution
done
clear
C)
free radical substitution
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 68) Which of the following is true at equilibrium?
A)
\[\Delta G=0\]
done
clear
B)
\[\Delta H=T\Delta S\]
done
clear
C)
\[\Delta G=\Delta H+T\Delta S\]
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 69) Dichlorobenzoic acid \[\xrightarrow{Mononitration}\]Product (only one). The structure of reactant can be
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 70) In the equilibrium \[2N{{H}_{3}}\rightleftarrows {{N}_{2}}+3{{H}_{2}},6\] moles of \[N{{H}_{3}}\] is taken in 10 L flask. If concentration of \[{{N}_{2}}\] at equilibrium is \[x\], then concentration of \[N{{H}_{3}}\] at equilibrium is
A)
\[0.6\,-x\]
done
clear
B)
\[0.6\,-2x\]
done
clear
C)
\[0.6\,-\frac{x}{2}\]
done
clear
D)
None is correct
done
clear
View Answer play_arrow
question_answer 71) Teflon is repeating unit of
A)
\[-C{{F}_{2}}-C{{F}_{2}}-\]
done
clear
B)
\[C{{F}_{2}}=C{{F}_{2}}\]
done
clear
C)
\[-(C{{H}_{2}}-C{{H}_{2}}){{-}_{n}}\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 72) The number of \[\pi \]-bonds in the following compound \[{{O}_{2}}N-C\equiv -N{{O}_{2}}\], is
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 73) Most reactive compound towards electrophilic substitution is
A)
done
clear
B)
done
clear
C)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 74) Which of the following is true representation of lattice energy?
A)
\[N{{a}^{+}}(s)+\frac{1}{2}C{{l}_{2}}(g)\xrightarrow{{}}NaCl(s)\]
done
clear
B)
\[N{{a}^{+}}(s)+C{{l}^{-}}(g)\xrightarrow{{}}NaCl(s)\]
done
clear
C)
\[N{{a}^{+}}(g)+C{{l}^{-}}(g)\xrightarrow{{}}NaCl(s)\]
done
clear
D)
\[N{{a}^{+}}(s)+Cl(s)\xrightarrow{{}}NaCl(s)\]
done
clear
View Answer play_arrow
question_answer 75)
Arrange the following acids in decreasing order of acidic strength \[\underset{I}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-COOH}}\,\] \[\underset{II}{\mathop{C{{H}_{3}}C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}OH}}\,\] \[\underset{\text{IV}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C\equiv CH}}\,\]
A)
\[I>II>III>IV\]
done
clear
B)
\[III>IV>II>I\]
done
clear
C)
\[I>III>IV>II\]
done
clear
D)
\[I>II>II>IV\]
done
clear
View Answer play_arrow
question_answer 76) The best condition for heterogeneous catalysis is
A)
adsorption
done
clear
B)
absorption
done
clear
C)
diffusion
done
clear
D)
occlusion
done
clear
View Answer play_arrow
question_answer 77) Functionality of protein depends on
A)
its shape and structure
done
clear
B)
pH of medium
done
clear
C)
temperature
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 78) In acidic medium, \[CrO_{4}^{2-}\] changes to
A)
\[C{{r}_{2}}O_{7}^{2-}\]
done
clear
B)
\[C{{r}^{3+}}\]
done
clear
C)
\[Cr\,(IV)\]
done
clear
D)
\[C{{r}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 79) \[F{{e}^{2+}}\xrightarrow{{}}F{{e}^{3+}}+{{e}^{-}}\]\[MnO_{4}^{-}+5{{e}^{-}}\xrightarrow{{}}M{{n}^{2+}}\]the ratio of stoichiometric coefficient of \[F{{e}^{2+}}\] and \[MnO_{4}^{-}\]is
A)
1 : 5
done
clear
B)
5 : 1
done
clear
C)
2 : 3
done
clear
D)
6 : 1
done
clear
View Answer play_arrow
question_answer 80)
Which of the following is change in enthalpy?
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
View Answer play_arrow
question_answer 81) \[Zn(s)+C{{l}_{2}}(1\,atm)\xrightarrow{{}}Z{{n}^{2+}}+2C{{l}^{-}}\]. \[E_{cell}^{o}\] of the cell is 2.12 V. To increase E
A)
\[[Z{{n}^{2+}}]\] should be increased
done
clear
B)
\[[Z{{n}^{2+}}]\] should be decreased
done
clear
C)
\[[C{{l}^{-}}]\] should be decreased
done
clear
D)
\[{{p}_{C{{l}_{2}}}}\] should be decreased
done
clear
View Answer play_arrow
question_answer 82) Which of the following substances acts as an oxidising as well as reducing agent?
A)
\[N{{a}_{2}}O\]
done
clear
B)
\[SnC{{l}_{2}}\]
done
clear
C)
\[N{{a}_{2}}{{O}_{2}}\]
done
clear
D)
\[NaN{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 83) Which one of the following ions has the highest value of ionic radius?
A)
\[{{O}^{2-}}\]
done
clear
B)
\[{{B}^{3+}}\]
done
clear
C)
\[L{{i}^{+}}\]
done
clear
D)
\[{{F}^{-}}\]
done
clear
View Answer play_arrow
question_answer 84) Setting of cement is an
A)
exothermic reaction
done
clear
B)
endothermic reaction
done
clear
C)
neither exothermic nor endothermic
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 85) Which of the following is paramagnetic?
A)
\[N{{i}^{2+}}\]
done
clear
B)
\[C{{u}^{+}}\]
done
clear
C)
\[Z{{n}^{2+}}\]
done
clear
D)
\[S{{c}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 86) Iron is rendered passive by the action of
A)
conc. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
cone. \[{{H}_{3}}P{{O}_{4}}\]
done
clear
C)
conc. \[HCl\]
done
clear
D)
conc. \[HN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 87) An aqueous solution of \[CoC{{l}_{2}}\] on addition of excess of cone. \[HCl\] turns blue due to the formation of
A)
\[[Co({{H}_{2}}O)]C{{l}_{2}}\]
done
clear
B)
\[[Co{{({{H}_{2}}O)}_{2}}]C{{l}_{4}}{{]}^{2-}}\]
done
clear
C)
\[{{[CoC{{l}_{4}}]}^{2-}}\]
done
clear
D)
\[[Co{{({{H}_{2}}O)}_{2}}C{{l}_{2}}]\]
done
clear
View Answer play_arrow
question_answer 88) Which of the following is not present in a nucleotide?
A)
Cytosine
done
clear
B)
Guanine
done
clear
C)
Adenine
done
clear
D)
Tyrosine
done
clear
View Answer play_arrow
question_answer 89) Which of the following is used as an antibiotic?
A)
Ciprofloxacin
done
clear
B)
Paracetamol
done
clear
C)
Ibuprofen
done
clear
D)
Tocopherol
done
clear
View Answer play_arrow
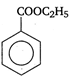
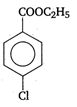
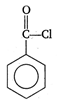
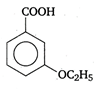
question_answer 90) Benzaldehyde condenses with N,N-Dimethylamline in presence of anhydrous \[ZnC{{l}_{2}}\] to give
A)
Michlers ketone
done
clear
B)
azo dye
done
clear
C)
malachite green
done
clear
D)
buffer yellow
done
clear
View Answer play_arrow
question_answer 91) Which of the following is secondary pollutant?
A)
\[C{{O}_{2}}\]
done
clear
B)
\[{{N}_{2}}O\]
done
clear
C)
PAN
done
clear
D)
\[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 92) What will be the volume of 12 M solution, if it is equivalent to 240 mL of 18 M solution?
A)
6L
done
clear
B)
600 L
done
clear
C)
400L
done
clear
D)
0.36L
done
clear
View Answer play_arrow
question_answer 93) The specific rotation of a pure enantiomer is\[+{{10}^{o}}\]. The observed rotation, if it is isolated from a reaction with 30% racemisation and 70% retention, is
A)
\[+{{10}^{o}}\]
done
clear
B)
\[+{{7}^{o}}\]
done
clear
C)
\[+{{14}^{o}}\]
done
clear
D)
\[-{{7}^{o}}\]
done
clear
View Answer play_arrow
question_answer 94) IUPAC name of the following \[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{N}}\,-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ {{C}_{2}}{{H}_{5}} \end{smallmatrix}}{\mathop{C}}\,}}\,-C{{H}_{2}}-C{{H}_{3}}\]
A)
3-dimethylamino-3-methyl pentane
done
clear
B)
3 (N,N-trimethyl)-3-amino pentane
done
clear
C)
3 N-N-trimethyl pentammine
done
clear
D)
3-(N,N-dimethyl) amino 3-methyl pentane
done
clear
View Answer play_arrow
question_answer 95) The equivalent weight of a metal is 9 and vapour density of its chloride is 59.25. The atomic weight of metal is
A)
23.9
done
clear
B)
27.3
done
clear
C)
36.3
done
clear
D)
48.3
done
clear
View Answer play_arrow
question_answer 96) Splitting of spectral lines under the influence of magnetic field is called
A)
Zeeman effect
done
clear
B)
Starck effect
done
clear
C)
Photoelectric effect
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 97) Which of the following molecule has pyramidal shape?
A)
\[PC{{l}_{3}}\]
done
clear
B)
\[S{{O}_{3}}\]
done
clear
C)
\[CO_{3}^{2-}\]
done
clear
D)
\[NO_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 98) Equation of Boyles law is
A)
\[\frac{dp}{p}=-\frac{dV}{V}\]
done
clear
B)
\[\frac{dp}{p}=+\frac{dV}{V}\]
done
clear
C)
\[\frac{{{d}^{2}}p}{p}=-\frac{dV}{V}\]
done
clear
D)
\[\frac{{{d}^{2}}p}{p}=+\frac{{{d}^{2}}V}{dT}\]
done
clear
View Answer play_arrow
question_answer 99) An element has half-life 1600 yr. The mass left after 6400 yr will be
A)
1/16
done
clear
B)
1/12
done
clear
C)
1/4
done
clear
D)
1/32
done
clear
View Answer play_arrow
question_answer 100) Boiling point of chloroform was raised by 0.323 K, when 0.5143 g of anthracene was dissolved in 35 g of chloroform. Molecular mass of anthracene is \[({{k}_{f}}for\,CHC{{l}_{3}}=3.9\,K\,kg\,mo{{l}^{-1}})\]
A)
79.42 g/mol
done
clear
B)
132.32 g/mol
done
clear
C)
177.42 g/mol
done
clear
D)
242.32 g/mol
done
clear
View Answer play_arrow
question_answer 101) Which of the following event occurs during \[{{G}_{1}}\]-phase?
A)
DNA replication
done
clear
B)
Growth and normal function of cell
done
clear
C)
Mutation
done
clear
D)
Fertilization
done
clear
View Answer play_arrow
question_answer 102) The stage during which, cell decides to get specialized
A)
S-phase
done
clear
B)
M-phase
done
clear
C)
\[{{G}_{1}}\]-phase
done
clear
D)
\[{{G}_{2}}\]-phase
done
clear
View Answer play_arrow
question_answer 103) In which of the following wavelength, photosystem-I is inactive?
A)
780 nm
done
clear
B)
680 nm
done
clear
C)
690 nm
done
clear
D)
550 nm
done
clear
View Answer play_arrow
question_answer 104) ADH deficiency causes
A)
diabetes insipidus
done
clear
B)
goitre
done
clear
C)
tetany
done
clear
D)
acromegaly
done
clear
View Answer play_arrow
question_answer 105) Law of limiting factor of photosynthesis was proposed by
A)
von Mayer
done
clear
B)
Arnon
done
clear
C)
FF Blackmann
done
clear
D)
Hill
done
clear
View Answer play_arrow
question_answer 106) Opening of stomaia is not affected by
A)
\[{{N}_{3}}\]
done
clear
B)
\[{{K}^{+}}\] ions
done
clear
C)
Starch
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 107) The IV cranial nerve is
A)
oculomotor
done
clear
B)
trochlear
done
clear
C)
olfactory
done
clear
D)
facial
done
clear
View Answer play_arrow
question_answer 108) Subunits of 80S ribosome are
A)
40S
done
clear
B)
60S
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 109) The stored food in animals is called
A)
cellulose
done
clear
B)
starch
done
clear
C)
glucose
done
clear
D)
glycogen
done
clear
View Answer play_arrow
question_answer 110) The cellular respiration first takes place in the
A)
cytoplasm
done
clear
B)
Golgi bodies
done
clear
C)
ER
done
clear
D)
lysosomes
done
clear
View Answer play_arrow
question_answer 111) Which of the following substrate is used in the formation of alcohol?
A)
Sucrose
done
clear
B)
Glucose
done
clear
C)
Galactose
done
clear
D)
Fructose
done
clear
View Answer play_arrow
question_answer 112) If a person is feeding only on meat, egg and milk then he will suffer from
A)
nightblindness
done
clear
B)
scurvy
done
clear
C)
rickets
done
clear
D)
beri-beri
done
clear
View Answer play_arrow
question_answer 113) Rubisco stands for
A)
Ribulose bisphosphate carboxylase oxygenase
done
clear
B)
Ribulose phosphate carboxylase xygenase
done
clear
C)
Ribulose phosphate carboxylic oxygenase
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 114) Net gain of ATP molecules per hexose during aerobic respiration is
A)
12
done
clear
B)
18
done
clear
C)
36
done
clear
D)
30
done
clear
View Answer play_arrow
question_answer 115) The term hot dilute soup was used by
A)
Haldane
done
clear
B)
von Helmont
done
clear
C)
Redi
done
clear
D)
Louis Pasteur
done
clear
View Answer play_arrow
question_answer 116) Which of the following classification is based on some morphological characters?
A)
Artificial
done
clear
B)
Natural
done
clear
C)
Phylogenetic
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 117) Sweet potato and potato are examples of
A)
homologous structure
done
clear
B)
analogous structure
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 118) Systema Naturae was written by
A)
Hutchinson
done
clear
B)
Lamarck
done
clear
C)
Linnaeus
done
clear
D)
Bentham and Hooker
done
clear
View Answer play_arrow
question_answer 119) In the origin of life, microspheres are moot primitive protobiont, which have a membrane of
A)
lipid and proteins
done
clear
B)
lipid
done
clear
C)
carbohydrates
done
clear
D)
fats
done
clear
View Answer play_arrow
question_answer 120) Rabies is caused by
A)
virus
done
clear
B)
bacteria
done
clear
C)
Protozoa
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 121) Tuberculosis is caused by
A)
Vibrio cholerae
done
clear
B)
Mycobacterium
done
clear
C)
Salmonella typhi
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 122) Fresh water bony fish survives by
A)
get rid of \[{{H}_{2}}O\] and gain salt
done
clear
B)
get rid of salt and gain \[{{H}_{2}}O\]
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 123) Nitrification is the process of conversion of
A)
\[N{{H}_{3}}\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[N{{O}_{3}}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 124) Which of the following provides nutrition tosperm?
A)
Leydig cells
done
clear
B)
Scrotum
done
clear
C)
Sertoli cells
done
clear
D)
Epididymis
done
clear
View Answer play_arrow
question_answer 125) Oral contraceptive pills function by
A)
fertilization
done
clear
B)
inhibiting ovulation
done
clear
C)
reproduction
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 126) Sphaerocarpos belongs to
A)
Bryophyta
done
clear
B)
Pteridophyta
done
clear
C)
gymnosperm
done
clear
D)
angiosperm
done
clear
View Answer play_arrow
question_answer 127) Fungi differs from slime moulds by lacking of
A)
flagellated spores
done
clear
B)
ascospores
done
clear
C)
basidiospores
done
clear
D)
zygospores
done
clear
View Answer play_arrow
question_answer 128) Saprozoic type of feeding habit is found in which of the following animal?
A)
Mosquito
done
clear
B)
Scorpion
done
clear
C)
Spider
done
clear
D)
Cockroach
done
clear
View Answer play_arrow
question_answer 129) Fungi are classified on the basis of
A)
sexual reproduction
done
clear
B)
asexual reproduction
done
clear
C)
vegetative reproduction
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 130) Wiich one of the following does not belong to kingdom-Monera?
A)
Mycoplasma
done
clear
B)
Archaebacteria
done
clear
C)
Slime mould
done
clear
D)
Eubacteria
done
clear
View Answer play_arrow
question_answer 131) Which one of the following is not a plastid?
A)
Mitoplast
done
clear
B)
Chromoplast
done
clear
C)
Chloroplast
done
clear
D)
Leucoplast
done
clear
View Answer play_arrow
question_answer 132) In retroviruses, RNA dependent DMA Polymerase synthesizes
A)
RNA-DNA
done
clear
B)
DNA
done
clear
C)
RNA
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 133) What will happen. When glucose is administered orally?
A)
Excretion
done
clear
B)
Digestion
done
clear
C)
Circulation
done
clear
D)
Respiration
done
clear
View Answer play_arrow
question_answer 134) Agrose is a gel, which is used to separate
A)
carbohydrate
done
clear
B)
fats
done
clear
C)
Both [a] and [b]
done
clear
D)
protein
done
clear
View Answer play_arrow
question_answer 135) The process of reverse transcription was discovered by
A)
Temin and Baltimore
done
clear
B)
Watson and Crick
done
clear
C)
Alfred Hershey
done
clear
D)
None of the aboce
done
clear
View Answer play_arrow
question_answer 136) Which one of the following animal has pseudocoelome ?
A)
Cockroach
done
clear
B)
Ancylostoma
done
clear
C)
Aurelia
done
clear
D)
Fasiola
done
clear
View Answer play_arrow
question_answer 137) The process of joining of amino acids is called
A)
transcription
done
clear
B)
translation
done
clear
C)
conjugation
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 138) Which of the following isotopes is used for finding the fossil having age more than 4500 year?
A)
\[{{U}^{238}}\]
done
clear
B)
\[{{U}^{235}}\]
done
clear
C)
\[P{{o}^{235}}\]
done
clear
D)
\[^{12}C\]
done
clear
View Answer play_arrow
question_answer 139) The infective stage of Plasmodium vivax is
A)
sporozoite
done
clear
B)
gametocyte
done
clear
C)
trophozoite
done
clear
D)
cryptozoite
done
clear
View Answer play_arrow
question_answer 140) Which of the following is obtained from genetic engineering?
A)
Haemoglobin
done
clear
B)
Glucose
done
clear
C)
Golden rice
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 141) H. Which of the following is a test cross?
A)
Ww \[\times \] ww
done
clear
B)
WW \[\times \] ww
done
clear
C)
Ww \[\times \] Ww
done
clear
D)
ww \[\times \] ww
done
clear
View Answer play_arrow
question_answer 142) One gene-one enzyme hypothesis wa propounded by
A)
Beadle and Tatum
done
clear
B)
Louis Pasteur
done
clear
C)
J B S Haldane
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 143) The glenoid cavity is associated with
A)
scapula
done
clear
B)
humerus
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 144) The nerve fibre in its resting stage is
A)
more permeable to \[{{K}^{+}}\]
done
clear
B)
semi-permeable to \[{{K}^{+}}\]
done
clear
C)
less permeable to \[{{K}^{+}}\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 145) Genes when close together on a chromosomes are known as
A)
linkage
done
clear
B)
mutation
done
clear
C)
translation
done
clear
D)
transcription
done
clear
View Answer play_arrow
question_answer 146) The theory of pangenesis was rejected due to the acceptance of
A)
Spallanzani theory of biogenesis
done
clear
B)
Richter theory of cosmozoic
done
clear
C)
Cuvier theory of catastropism
done
clear
D)
Weismann theory of germplasm
done
clear
View Answer play_arrow
question_answer 147) In human beings, 45 chromosomes/single X/XO abnormality causes
A)
Downs syndrome
done
clear
B)
Klinefelters syndrome
done
clear
C)
Turners syndrome
done
clear
D)
Edwards syndrome
done
clear
View Answer play_arrow
question_answer 148) An evolutionary process, giving rise to new species adapting to new habitats and ways of life is called
A)
adaptive radiation
done
clear
B)
adaptation
done
clear
C)
convergent evolution
done
clear
D)
microevolution
done
clear
View Answer play_arrow
question_answer 149) The chromosomal arrangement results in
A)
euploidy
done
clear
B)
aneuploidy
done
clear
C)
duplication
done
clear
D)
polyploidy
done
clear
View Answer play_arrow
question_answer 150) One of these is associated with terminator codon?
A)
AGG
done
clear
B)
UAA
done
clear
C)
UUA
done
clear
D)
AUG
done
clear
View Answer play_arrow
question_answer 151) The inherent maximum capacity of an organism to reproduce or increase in number is called as
A)
biotic potential
done
clear
B)
ecosystem
done
clear
C)
population
done
clear
D)
ecology
done
clear
View Answer play_arrow
question_answer 152) Which of the following is best suited for co dominance?
A)
Both are recessive
done
clear
B)
Both are dominance
done
clear
C)
One is recessive
done
clear
D)
One is dominance
done
clear
View Answer play_arrow
question_answer 153) The substance which is metal ion for the normal functioning of enzyme is called
A)
cofactor
done
clear
B)
coenzyme
done
clear
C)
holoenzyme
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 154) Scala Naturae was written by
A)
Linnaeus
done
clear
B)
Darwin
done
clear
C)
Aristotle
done
clear
D)
Whittaker
done
clear
View Answer play_arrow
question_answer 155) The gene which masks the effect of another gene is called
A)
epistasis
done
clear
B)
lethal gene
done
clear
C)
multiple allele
done
clear
D)
complementary gene
done
clear
View Answer play_arrow
question_answer 156) In a DNA molecule, the adenine is 15%. Wha will be the percentage of guanine in this DNA?
A)
15%
done
clear
B)
35%
done
clear
C)
70%
done
clear
D)
30%
done
clear
View Answer play_arrow
question_answer 157) Which one is used in the production of insulin by genetic engineering?
A)
Eschereria coli
done
clear
B)
Mycobacterium
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 158) The synthesis of complex molecules from simple molecules was proved by
A)
Arrhenius
done
clear
B)
Pasteur
done
clear
C)
Stanley Miller
done
clear
D)
Redi
done
clear
View Answer play_arrow
question_answer 159) Which is not correct according to Chargaffs rule?
A)
\[A+T=C+G\]
done
clear
B)
\[A+G=C+T\]
done
clear
C)
\[\frac{A+G}{C+T}=1\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 160) The interaction of species with the environment is called as
A)
community
done
clear
B)
environment
done
clear
C)
ecosystem
done
clear
D)
autecology
done
clear
View Answer play_arrow
question_answer 161) Which of the following cells are associated with identification of colours in bright light?
A)
Rod cells
done
clear
B)
Cone cells
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 162) Which of the following defines Hardey Weinbergs law?
A)
\[{{p}^{2}}+\text{ }2pq+\text{ }{{q}^{2}}=\text{ }1\]
done
clear
B)
\[{{p}^{2}}+\text{ }2pq+\text{ }{{q}^{2}}=\text{ }1\]
done
clear
C)
\[{{p}^{2}}+\text{ }2pq+\text{ }{{q}^{2}}=\text{ }0\]
done
clear
D)
\[{{q}^{2}}+\text{ }{{p}^{2}}+\text{ }2pq=\text{ }0\]
done
clear
View Answer play_arrow
question_answer 163) The size of the clay particle is less than
A)
0.02 mm
done
clear
B)
0.002 mm
done
clear
C)
0.2 mm
done
clear
D)
2.0 mm
done
clear
View Answer play_arrow
question_answer 164) Photochemical smog pollution does not contain
A)
ozone
done
clear
B)
nitrogen dioxide
done
clear
C)
carbon dioxide
done
clear
D)
PAN
done
clear
View Answer play_arrow
question_answer 165) . Decrease in the calcium level in blood is caused by
A)
prolactin
done
clear
B)
calcitonin
done
clear
C)
adrenocorticotropin
done
clear
D)
oxytocin
done
clear
View Answer play_arrow
question_answer 166) Heavier and lighter isotopic form of an element can be separated by the technique of
A)
buoyant density centrifugation
done
clear
B)
density gradient centrifugation
done
clear
C)
chromatography
done
clear
D)
electrophoresis
done
clear
View Answer play_arrow
question_answer 167) The ozone layer is found in
A)
troposphere
done
clear
B)
mesosphere
done
clear
C)
stratosphere
done
clear
D)
atmosphere
done
clear
View Answer play_arrow
question_answer 168) The pectoral fins get enlarged in
A)
Exocoetus
done
clear
B)
Scoliodon
done
clear
C)
Hippocampus
done
clear
D)
Coccosreus
done
clear
View Answer play_arrow
question_answer 169) What is a keystone species ?
A)
A species which adds upto only a small proportion of the total biomass of a community, yet has a huge impact on the communitys organization and survival
done
clear
B)
A common species that has plenty of biomass, yet has a fairly low impact on the communitys organization
done
clear
C)
A rare species that has minimal impact oc the biomass and on other species in the community
done
clear
D)
A dominant species that constitutes a large proportion of the biomass and which affects many other species
done
clear
View Answer play_arrow
question_answer 170) Which one of the following plant functions as symbiotic nitrogen fixing plant?
A)
Azolla
done
clear
B)
Cycas
done
clear
C)
Moss
done
clear
D)
Marchantia
done
clear
View Answer play_arrow
question_answer 171) Bowmans glands are found in
A)
olfactory epithelium
done
clear
B)
external auditory canal
done
clear
C)
cortical nephrons only
done
clear
D)
juxta medullary nephrons
done
clear
View Answer play_arrow
question_answer 172) An important evidence in favour of organic evolution is the occurrence of
A)
homologous and vestigial organs
done
clear
B)
analogous and vestigial organs
done
clear
C)
homologous organs only
done
clear
D)
Homologous and analogous organs
done
clear
View Answer play_arrow
question_answer 173) Eutrophication is the result of
A)
bryophyte
done
clear
B)
algae and aquatic plants
done
clear
C)
gymnosperm
done
clear
D)
pteridophyte
done
clear
View Answer play_arrow
question_answer 174) Which one of the following is no biodegradable?
A)
Sewage
done
clear
B)
DDT
done
clear
C)
Live stock waste
done
clear
D)
Market garbage
done
clear
View Answer play_arrow
question_answer 175) ADH controls water permeability of
A)
distal convoluted tubule
done
clear
B)
proximal convoluted tubule
done
clear
C)
Bowmans capsule
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 176) The cause of decline in the population of reptiles and birds is
A)
DDT
done
clear
B)
biofertilizer
done
clear
C)
bioinsecticides
done
clear
D)
biodegradable
done
clear
View Answer play_arrow
question_answer 177) Which one of the following shows detritus food Aain?
A)
Organic waste \[\to \] Bacteria \[\to \] Molluscus
done
clear
B)
Grass \[\to \] Insects \[\to \] Snakes
done
clear
C)
Plankton \[\to \] Small fishes \[\to \] Large fishes
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 178) Which of the following enzyme helps in digesting protein in stomach?
A)
Trypsin
done
clear
B)
Pepsin
done
clear
C)
Ptyalin
done
clear
D)
Rennin
done
clear
View Answer play_arrow
question_answer 179) The Leydig cells secrete
A)
estrogen
done
clear
B)
testosterone
done
clear
C)
progesterone
done
clear
D)
cortisol
done
clear
View Answer play_arrow
question_answer 180) Which one of the following is not a biofertilizer?
A)
Bacillus thuringiensis
done
clear
B)
Azotobacter
done
clear
C)
Azolla
done
clear
D)
Clostridium
done
clear
View Answer play_arrow
question_answer 181) In India hot spot area is found in
A)
Western Himalaya
done
clear
B)
Tropical Andes
done
clear
C)
Madagascar
done
clear
D)
Mesoamerica
done
clear
View Answer play_arrow
question_answer 182) The thalloid body of a slime mould (Myxomycetes) is known as
A)
protonema
done
clear
B)
plasmodium
done
clear
C)
fruiting body
done
clear
D)
mycelium
done
clear
View Answer play_arrow
question_answer 183) A normal woman whose father was a colour blind person is married to a normal man. Her sons would likely to be
A)
75% colourblind
done
clear
B)
50% colourblind
done
clear
C)
All normal
done
clear
D)
All colourblind
done
clear
View Answer play_arrow
question_answer 184) Which of the following is not a function of liver?
A)
Production of bile
done
clear
B)
Production of insulin
done
clear
C)
Glycogen storage
done
clear
D)
Detoxification
done
clear
View Answer play_arrow
question_answer 185) The vital capacity of human lung is equal to
A)
500 mL
done
clear
B)
4600 mL
done
clear
C)
5800 mL
done
clear
D)
2300 Ml
done
clear
View Answer play_arrow
question_answer 186) Wavelength of visibile light/PAR is
A)
200-400 nm
done
clear
B)
700-900 nm
done
clear
C)
400-700 nm
done
clear
D)
100-200 nm
done
clear
View Answer play_arrow
question_answer 187) During day time sound level in silent zone is
A)
50 dB
done
clear
B)
70 dB
done
clear
C)
20 dB
done
clear
D)
40 dB
done
clear
View Answer play_arrow
question_answer 188) DNA can be formed by
A)
transaminase
done
clear
B)
lyases
done
clear
C)
RNA dependent DNA polymerase
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 189) A cell active in protein synthesis will rich in
A)
ribosomes
done
clear
B)
Golgi bodies
done
clear
C)
mitochondria
done
clear
D)
lysosomes
done
clear
View Answer play_arrow
question_answer 190) The RNA primer is used in
A)
translation
done
clear
B)
replication
done
clear
C)
conjugation
done
clear
D)
transformation
done
clear
View Answer play_arrow
question_answer 191) The yellow colour of urine is caused by
A)
urochrome
done
clear
B)
bilirubin
done
clear
C)
Biliverdin
done
clear
D)
xanthophylls
done
clear
View Answer play_arrow
question_answer 192) The number of species classified in Species Plantarum
A)
5900
done
clear
B)
6000
done
clear
C)
4000
done
clear
D)
3800
done
clear
View Answer play_arrow
question_answer 193) Sigmoid notch is formed by
A)
cavity formed by humerus
done
clear
B)
cavity formed by radio-ulna
done
clear
C)
cavity formed by tibio-fibula
done
clear
D)
cavity formed by femur
done
clear
View Answer play_arrow
question_answer 194) The term innominate is related with
A)
nerve
done
clear
B)
artery
done
clear
C)
skeleton
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 195) Meselson Stahl experiment on semi- conservative replication demonstrates that
A)
50% radioactive, 50% non-radioactive
done
clear
B)
50% non-radioactive
done
clear
C)
50% radioactive
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 196) Characteristic cells of Hydra are
A)
archeocytes
done
clear
B)
thesocytes
done
clear
C)
cnidoblasts
done
clear
D)
trophocytes
done
clear
View Answer play_arrow
question_answer 197) An acoelomate animal with bilateral symmetry is
A)
Hydra
done
clear
B)
Liver fluke
done
clear
C)
Physalia
done
clear
D)
Obelia
done
clear
View Answer play_arrow
question_answer 198) The opening of auricles and ventricle on the right side is guarded by
A)
tricuspid valve
done
clear
B)
bicuspid valve
done
clear
C)
semilunar valve
done
clear
D)
eustachian tube
done
clear
View Answer play_arrow
question_answer 199) In \[{{F}_{2}}\]-generation, quantitation inheritance 1 : 4 : 6 : 4 : 1 is obtained instead of
A)
9 : 3 : 3 : 1
done
clear
B)
8 : 6 : 4 : 1
done
clear
C)
7 : 4 : 1 : 4
done
clear
D)
6 : 6 : 4 : 7
done
clear
View Answer play_arrow
question_answer 200) Incomplete dominance is shown by
A)
Primrose
done
clear
B)
Mirabilis
done
clear
C)
Helianthus
done
clear
D)
China rose
done
clear
View Answer play_arrow
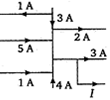 What is current \[I.\]
What is current \[I.\]
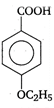
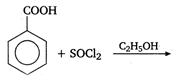
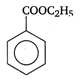
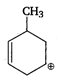
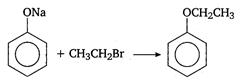 the type of reaction is
the type of reaction is