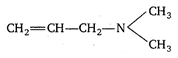question_answer 1) A Carnots engine has an efficiency of 50% at sink temperature\[{{50}^{o}}C\]. Calculate the temperature of source.
A)
\[{{133}^{o}}C\]
done
clear
B)
\[{{143}^{o}}C\]
done
clear
C)
\[{{100}^{o}}C\]
done
clear
D)
\[{{373}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 2) Which of the following is not a state function?
A)
Work done at constant pressure
done
clear
B)
Enthalpy
done
clear
C)
Work done by conservative force
done
clear
D)
Work done by non-conservative force
done
clear
View Answer play_arrow
question_answer 3) what is the Q-value of the reaction \[p{{+}^{7}}Li{{\xrightarrow{{}}}^{4}}He{{+}^{4}}He\] The atomic masses of \[^{1}H{{,}^{4}}He\] and \[^{7}Li\] are 1.007825 u, 4.002603 u and 7.016004 u respectively
A)
17.35 MeV
done
clear
B)
18.06 MeV
done
clear
C)
177.35 MeV
done
clear
D)
170.35 MeV
done
clear
View Answer play_arrow
question_answer 4) If average velocity becomes 4 times then t-hat will be the effect on rms velocity at that Temperature?
A)
1.4 times
done
clear
B)
4 times
done
clear
C)
2 times
done
clear
D)
\[\frac{1}{4}\]times
done
clear
View Answer play_arrow
question_answer 5) An aluminium rod and a copper rod are taken such that their lengths are same and their resistances are also same. The specific, resistance of copper is half that of aluminium, but its density is three times that of aluminium. The ratio of the mass of aluminium rod and that of copper rod will be
A)
\[\frac{1}{6}\]
done
clear
B)
\[\frac{2}{3}\]
done
clear
C)
\[\frac{1}{3}\]
done
clear
D)
\[6\]
done
clear
View Answer play_arrow
question_answer 6) In a potentiometer the null point is received at 7th wire. If now we have to change the null point at 9th wire, what should we do?
A)
Attach resistance in series with battery
done
clear
B)
Increase resistance in main circuit
done
clear
C)
Decrease resistance in main circuit
done
clear
D)
Decrease applied emf
done
clear
View Answer play_arrow
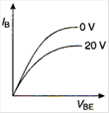
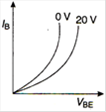
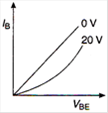
question_answer 7) Graph of input characteristic of common emitter amplifier is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
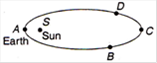
question_answer 8)
The earth moves in an elliptical orbit with the sun S at one of foci as shown in the figure. Its rotational kinetic energy is maximum at the point
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
View Answer play_arrow
question_answer 9) If \[{{B}_{H}}=\frac{1}{\sqrt{3}}{{B}_{V,}}\] find angle of dip. (where symbols have their usual meanings)
A)
\[{{60}^{o}}\]
done
clear
B)
\[{{30}^{o}}\]
done
clear
C)
\[{{45}^{o}}\]
done
clear
D)
\[{{90}^{o}}\]
done
clear
View Answer play_arrow
question_answer 10) Reverberation time does not depend upon
A)
temperature
done
clear
B)
volume of room
done
clear
C)
size of window
done
clear
D)
carpet and curtain
done
clear
View Answer play_arrow
question_answer 11) The output is low when either of the input is high, then this represents which of the following gates?
A)
OR
done
clear
B)
NOR
done
clear
C)
AND
done
clear
D)
NAND
done
clear
View Answer play_arrow
question_answer 12) In a semiconductor electron concentration is \[7\times {{10}^{13}}\,c{{m}^{-3}}\] and hole concentration is\[5\times {{10}^{12}}\,c{{m}^{-3}}.\]Then the semiconductor is
A)
n-type
done
clear
B)
p-type
done
clear
C)
intrinsic
done
clear
D)
p-n type
done
clear
View Answer play_arrow
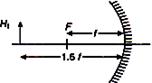
question_answer 13)
If in the following figure, height of object is\[{{H}_{1}}=+2.5\,cm\], then height of image \[{{H}_{2}}\]formed is
A)
-5 cm
done
clear
B)
+5 cm
done
clear
C)
+7.5 cm
done
clear
D)
-7.5 cm
done
clear
View Answer play_arrow
question_answer 14) Moment of inertia of ring about its diameter is \[I\] Then, moment of inertia about an axis passing through centre perpendicular to its plane is
A)
\[2I\]
done
clear
B)
\[\frac{I}{2}\]
done
clear
C)
\[\frac{3}{2}I\]
done
clear
D)
\[I\]
done
clear
View Answer play_arrow
question_answer 15) Which of the following is true regarding beats?
A)
Frequency different, amplitude same
done
clear
B)
Frequency same, amplitude same
done
clear
C)
Frequency same, amplitude different
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 16) When both the listener and source are moving towards each other, then which of the following is true regarding frequency and wavelength of wave observed by the observer?
A)
More frequency, less wavelength
done
clear
B)
More frequency, more wavelength
done
clear
C)
Less frequency, less wavelength
done
clear
D)
More frequency, constant wavelength
done
clear
View Answer play_arrow
question_answer 17) Which motion does not require force to maintain it?
A)
Uniform circular motion
done
clear
B)
Elliptical motion
done
clear
C)
Uniform straight line motion
done
clear
D)
Projectile motion
done
clear
View Answer play_arrow
question_answer 18) The coefficient of friction between the tyres and the road is 0.25. The maximum speed with which car can be driven round a curve of radius 40 m without skidding is (assume\[g=10\,\,s{{m}^{-2}}\])
A)
\[40\,\,m{{s}^{-1}}\]
done
clear
B)
\[20\,\,m{{s}^{-1}}\]
done
clear
C)
\[15\,\,m{{s}^{-1}}\]
done
clear
D)
\[10\,\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 19) In forward bias the width of depletion layer is
A)
decreases with increase in potential barrier voltage
done
clear
B)
increases with increase in potential barrier voltage
done
clear
C)
independent of potential barrier voltage
done
clear
D)
none of the above
done
clear
View Answer play_arrow
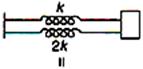
question_answer 20)
If \[{{k}_{s}}\] and \[{{k}_{p}}\] respectively are effective spring constant in series and parallel combination of of springs as shown in figure, find \[\frac{{{k}_{s}}}{{{k}_{p}}.}\]
A)
\[\frac{9}{2}\]
done
clear
B)
\[\frac{3}{7}\]
done
clear
C)
\[\frac{2}{9}\]
done
clear
D)
\[\frac{7}{3}\]
done
clear
View Answer play_arrow
question_answer 21)
A bob of pendulum was filled with Hg and entire Hg is drained out, then the time period of pendulum
A)
remains unchanged
done
clear
B)
decreases
done
clear
C)
increases
done
clear
D)
increases then decreases
done
clear
View Answer play_arrow
question_answer 22) The minimum force required to move a body of mass m vertically upward is
A)
a mg
done
clear
B)
\[mg/2\]
done
clear
C)
more than 2mg
done
clear
D)
more than mg
done
clear
View Answer play_arrow
question_answer 23) The uncertainty in the momentum of a particle is\[{{10}^{-30}}kg-m/s\]. The minimum uncertainty in its position will be
A)
\[{{10}^{-8}}m\]
done
clear
B)
\[{{10}^{-12}}m\]
done
clear
C)
\[{{10}^{-16}}m\]
done
clear
D)
\[{{10}^{-4}}m\]
done
clear
View Answer play_arrow
question_answer 24) If distance between earth and sun become four times then time period becomes
A)
4 times
done
clear
B)
8 times
done
clear
C)
1/4 times
done
clear
D)
1/8 times
done
clear
View Answer play_arrow
question_answer 25)
An air bubble is contained inside water. It behaves as a
A)
concave lens
done
clear
B)
convex lens
done
clear
C)
neither convex nor concave
done
clear
D)
cannot say
done
clear
View Answer play_arrow
question_answer 26) The power dissipated across resistance R which is connected across a battery of potential V is P. If resistance is doubled, then the power becomes
A)
\[\frac{1}{2}\]
done
clear
B)
\[2\]
done
clear
C)
\[\frac{1}{4}\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 27) BE/nucleon relation with mass number
A)
first decreases then increases
done
clear
B)
first increases then decreases
done
clear
C)
increases
done
clear
D)
decreases
done
clear
View Answer play_arrow
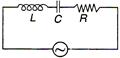
question_answer 28)
A 100 V, AC source of frequency 500 Hz is connected to an LCR circuit with \[L=8.1\,mH\], \[C=12.5\mu F,R=10\,\Omega \] all connected in series as shown in figure. What is the quality factor of circuit?
A)
2.02
done
clear
B)
2.5434
done
clear
C)
20.54
done
clear
D)
200.54
done
clear
View Answer play_arrow
question_answer 29) The inductance of a coil is \[L=10\,H\]and resistance \[R=5\,\Omega \]. If applied voltage of battery is 10 V and it switches off in 1 millisecond, find induced emf of inductor.
A)
\[2\times {{10}^{4}}V\]
done
clear
B)
\[1.2\times {{10}^{4}}V\]
done
clear
C)
\[2\times {{10}^{-4}}V\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 30) Two bodies A and B having masses in the ratio of \[3:1\] possess the same kinetic energy. The ratio of linear momentum of B to A is
A)
\[1:3\]
done
clear
B)
\[3:1\]
done
clear
C)
\[1:\sqrt{3}\]
done
clear
D)
\[\sqrt{3}:1\]
done
clear
View Answer play_arrow
question_answer 31) Which of the following four fundamental forces has shortest range?
A)
Nuclear
done
clear
B)
Gravitational
done
clear
C)
Electromagnetic
done
clear
D)
Weak force
done
clear
View Answer play_arrow
question_answer 32) A proton is moving in a uniform magnetic field B in a circular path of radius a in a direction perpendicular to z-axis along which field B exists. Calculate the angular momentum, if the radius is a charge on proton is e.
A)
\[\frac{Be}{{{a}^{2}}}\]
done
clear
B)
\[e{{B}^{2}}a\]
done
clear
C)
\[{{a}^{2}}eB\]
done
clear
D)
\[aeB\]
done
clear
View Answer play_arrow
question_answer 33) If acceleration of a particle at any time is given by \[a=2t\text{ }+5\] calculate the velocity after 5 s, if it starts from rest.
A)
50 m/s
done
clear
B)
25 m/s
done
clear
C)
100 m/s
done
clear
D)
75 m/s
done
clear
View Answer play_arrow
question_answer 34) 8 bits form
A)
1 byte
done
clear
B)
1 diction
done
clear
C)
1 nibble
done
clear
D)
1 binary
done
clear
View Answer play_arrow
question_answer 35) A body from height h is dropped. If the coefficient of restitution is e, then calculate the height achieved after one bounce.
A)
\[{{h}_{1}}={{e}^{2}}\,h\]
done
clear
B)
\[{{h}_{1}}={{e}^{4}}\,h\]
done
clear
C)
\[{{h}_{1}}=eh\]
done
clear
D)
\[{{h}_{1}}=\frac{h}{e}\]
done
clear
View Answer play_arrow
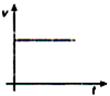
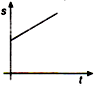
question_answer 36) A body moves with uniform acceleration, then which of the following graphs is correct?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 37) Two vectors are perpendicular, if
A)
\[\vec{A}\,.\,\vec{B}=1\]
done
clear
B)
\[\vec{A}\,\,\times \,\vec{B}=0\]
done
clear
C)
\[\vec{A}\,\,.\,\vec{B}=0\]
done
clear
D)
\[\vec{A}\,\,\times \,\vec{B}=AB\]
done
clear
View Answer play_arrow
question_answer 38) If coefficient of static friction is \[{{\mu }_{s}}\] and coefficient of kinetic friction is \[{{\mu }_{k}}\], which is correct?
A)
\[{{\mu }_{s}}={{\mu }_{k}}\]
done
clear
B)
\[{{\mu }_{s}}>{{\mu }_{k}}\]
done
clear
C)
\[{{\mu }_{s}}<{{\mu }_{k}}\]
done
clear
D)
Cannot predict
done
clear
View Answer play_arrow
question_answer 39) Light year is used to measure
A)
distance between stars
done
clear
B)
distance between atoms
done
clear
C)
revolution time of earth around sun
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 40) Electromagnetic waves are produced by
A)
accelerated charged particle
done
clear
B)
decelerated charged panicle
done
clear
C)
charge in uniform motion
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 41) A beam of protons with velocity \[4\times {{10}^{5}}m/s\]enters a uniform magnetic field of 0.3 T at an. angle of \[{{60}^{o}}\] to the magnetic field. Find the radius of the helical path taken by the proton beam.
A)
0.2cm
done
clear
B)
1.2cm
done
clear
C)
2.2cm
done
clear
D)
0.122cm
done
clear
View Answer play_arrow
question_answer 42)
Three progressive waves A, B and C are shown in the figure.
A)
B lags by \[\frac{\pi }{2}\] and C leads by \[\frac{\pi }{2}\]
done
clear
B)
B lags by \[\pi \] and C leads by \[\pi \]
done
clear
C)
B leads by \[\frac{\pi }{2}\] and C lags by \[\frac{\pi }{2}\]
done
clear
D)
B leads by n and C lags by \[\pi \]
done
clear
View Answer play_arrow
question_answer 43) When light passes from one medium to other then which will not change?
A)
Frequency
done
clear
B)
Wavelength
done
clear
C)
Amplitude
done
clear
D)
Velocity
done
clear
View Answer play_arrow
question_answer 44) Total internal reflection takes place
A)
when a ray moves from denser to rarer and incident angle is greater than critical angle
done
clear
B)
when a ray moves from rarer to denser and incident angle is less than critical angle
done
clear
C)
when a ray moves from rarer to denser and incident angle is equal to critical angle
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 45) A beam of light travelling along x-axis is described by the electric field \[{{E}_{y}}=600\text{ }V{{m}^{-1}}\text{ }sin~\,\omega \,\,(t-x/c)\]then maximum magnetic force on a charge\[q=2e\], moving along .y-axis with a speed of \[3.0\times {{10}^{7}}m{{s}^{-1}}\] is \[(e=1.6\times {{10}^{-19}}C)\]
A)
\[19.2\times {{10}^{-17}}N\]
done
clear
B)
\[1.92\times {{10}^{-17}}N\]
done
clear
C)
\[0.192\,\,N\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 46) In hydrogen atom spectrum, frequency of \[2.7\times {{10}^{15}}Hz\]of electromagnetic wave is emitted when transmission takes place from 2 to 1. If it moves from 3 to 1, the frequency emitted will be
A)
\[3.2\times {{10}^{15}}Hz\]
done
clear
B)
\[32\times {{10}^{15}}Hz\]
done
clear
C)
\[1.6\times {{10}^{15}}Hz\]
done
clear
D)
\[16\times {{10}^{15}}Hz\]
done
clear
View Answer play_arrow
question_answer 47) Potential drop across forward junction p-n diode is 0.7 V. If a battery of 4 V is applied, calculate the resistance to be put in series, if the maximum current in the circuit is 5mA.
A)
\[660\,\,\Omega \]
done
clear
B)
\[350\,\,\Omega \]
done
clear
C)
\[475\,\,\Omega \]
done
clear
D)
\[500\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 48) If 200MeV energy is released in the fission of a single nucleus of \[_{92}{{U}^{235}}\], how many fissions must occur per second to produce a power of 1kW?
A)
\[3.12\times {{10}^{13}}\]
done
clear
B)
\[3.12\times {{10}^{3}}\]
done
clear
C)
\[3.1\times {{10}^{17}}\]
done
clear
D)
\[3.12\times {{10}^{19}}\]
done
clear
View Answer play_arrow
question_answer 49) During projectile motion, the horizontal velocity
A)
first increases then decreases
done
clear
B)
first decreases then increases
done
clear
C)
always increases
done
clear
D)
always constant
done
clear
View Answer play_arrow
question_answer 50) Find ratio of acceleration due to gravity g at depth d and at height h, where d = 2h.
A)
\[1:1\]
done
clear
B)
\[1:2\]
done
clear
C)
\[2:1\]
done
clear
D)
\[1:4\]
done
clear
View Answer play_arrow
question_answer 51) Which of the following is polar?
A)
\[I_{3}^{-}\]
done
clear
B)
\[CO_{3}^{2-}\]
done
clear
C)
\[Xe{{F}_{4}}\]
done
clear
D)
\[P{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 52)
In the reaction
A)
\[{{H}_{3}}P{{O}_{2}}\]
done
clear
B)
\[C{{u}_{2}}C{{l}_{2}}\]
done
clear
C)
\[HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[{{H}^{+}}/{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 53) Ag crystallises as fee. If radius of Ag is 144 pm then its density will be
A)
10 g \[c{{m}^{-3}}\]
done
clear
B)
5g \[c{{m}^{-3}}\]
done
clear
C)
15 g \[c{{m}^{-3}}\]
done
clear
D)
6.5 g \[c{{m}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 54) Which of the following process will give soap?
A)
Hydrolysis
done
clear
B)
Saponification
done
clear
C)
Neutralisation
done
clear
D)
Acidification
done
clear
View Answer play_arrow
question_answer 55) Sodium hypohalite when dissolved in water will turn
A)
blue litmus red
done
clear
B)
red litmus blue
done
clear
C)
red litmus green
done
clear
D)
no change
done
clear
View Answer play_arrow
question_answer 56) A when added to silica will give B A and B are
A)
\[HF,{{H}_{2}}Si{{F}_{4}}\]
done
clear
B)
\[HF,{{H}_{2}}Si{{F}_{6}}\]
done
clear
C)
\[HCl,{{H}_{2}}SiC{{l}_{6}}\]
done
clear
D)
\[HI,{{H}_{2}}Si{{I}_{6}}\]
done
clear
View Answer play_arrow
question_answer 57)
The compound
A)
alkyne, \[{{3}^{o}}\] amine
done
clear
B)
alkene, \[{{2}^{o}}\]amine
done
clear
C)
alkene, \[{{3}^{o}}\] amine
done
clear
D)
alkyne, \[{{2}^{o}}\] amine
done
clear
View Answer play_arrow
question_answer 58) The number of geometrical isomers in \[C{{H}_{3}}CH=CHC{{H}_{2}}CH=C{{H}_{2}}\] are
A)
two
done
clear
B)
three
done
clear
C)
four
done
clear
D)
five
done
clear
View Answer play_arrow
question_answer 59) IUPAC name of the compound \[C{{H}_{2}}=CH\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,H-\underset{\begin{smallmatrix} | \\ {{C}_{2}}{{H}_{5}} \end{smallmatrix}}{\mathop{C}}\,H-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,HC{{H}_{3}}\]
A)
3-ethyl-4-methyl hex-5-en-2-ol
done
clear
B)
3-methyl-4-ethyl hex-l-en-5-ol
done
clear
C)
3-ethyl-2-hydroxy-4-methyl hex-5-ene
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 60) In the reaction \[C{{H}_{3}}C{{H}_{2}}COON{{H}_{4}}\xrightarrow{{{P}_{2}}{{O}_{5}}}A\xrightarrow{{{H}^{+}}/{{H}_{2}}O}B\] A and B are
A)
\[C{{H}_{3}}C{{H}_{2}}CON{{H}_{2}},C{{H}_{3}}C{{H}_{2}}CO{{O}^{-}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}COON{{H}_{2}},C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CN,C{{H}_{3}},C{{H}_{2}}CO{{O}^{-}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}CN,C{{H}_{3}},C{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 61) Number of a and n bonds in \[{{C}_{6}}{{H}_{5}}COOH\] are
A)
\[15\sigma ,\,4\pi \]
done
clear
B)
\[14\sigma ,\,4\pi \]
done
clear
C)
\[13\sigma ,\,4\pi \]
done
clear
D)
\[16\sigma ,\,4\pi \]
done
clear
View Answer play_arrow
question_answer 62) \[{{\Psi }^{2}}=0\] represent
A)
node
done
clear
B)
orbital
done
clear
C)
angular wave function
done
clear
D)
wave functions
done
clear
View Answer play_arrow
question_answer 63) Which of the following disaccharide has different type of linkage?
A)
Maltose
done
clear
B)
Galactose
done
clear
C)
Starch
done
clear
D)
Sucrose
done
clear
View Answer play_arrow
question_answer 64) For a reaction \[\Delta H=+29\,kJ\,mo{{l}^{-1}}\] \[\Delta S=-35\,kJ\,mo{{l}^{-1}}\] at what temperature the reaction will be spontaneous?
A)
\[{{828.7}^{o}}C\]
done
clear
B)
\[828.7K\]
done
clear
C)
spontaneous at all temperature
done
clear
D)
not possible
done
clear
View Answer play_arrow
question_answer 65) As the number of nucleon increases, binding energy per nucleon
A)
increases
done
clear
B)
decreases
done
clear
C)
first increases then decreases
done
clear
D)
first decreases then increases
done
clear
View Answer play_arrow
question_answer 66) Rate constant has the unit \[mo{{l}^{-2}}{{L}^{2}}{{s}^{-1}}\], then order of reaction is
A)
zero
done
clear
B)
first
done
clear
C)
second
done
clear
D)
third
done
clear
View Answer play_arrow
question_answer 67) The reaction follows the mechanism \[A+BAB\] (fast) \[AB+B\xrightarrow{{{k}_{2}}}A+{{B}_{2}}\] (slow) then rate law is
A)
\[r=k[A]\,[B]\]
done
clear
B)
\[r=k[AB]\,[B]\]
done
clear
C)
\[r=[A]\,{{[B]}^{2}}\]
done
clear
D)
\[r=k{{[A]}^{2}}\,[B]\]
done
clear
View Answer play_arrow
question_answer 68) Ozonolysis of which of the following will give HCHO?
A)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
D)
\[C{{H}_{2}}=CHCH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 69) 25g of \[MC{{l}_{4}}\] contains 0.5 mole chlorine then its molecular weight is
A)
100 g \[mo{{l}^{-1}}\]
done
clear
B)
200 g \[mo{{l}^{-1}}\]
done
clear
C)
150 g \[mo{{l}^{-1}}\]
done
clear
D)
400 g \[mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 70) pH of the solution containing \[1.04\times {{10}^{-12}}M\]KOH.is
A)
-11.9
done
clear
B)
+11.9
done
clear
C)
7
done
clear
D)
2.30
done
clear
View Answer play_arrow
question_answer 71) Benzene gives mainly
A)
addition reaction
done
clear
B)
elimination reaction
done
clear
C)
substitution reaction
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 72) Formula of ammonium manganate is
A)
\[N{{H}_{4}}Mn{{O}_{4}}\]
done
clear
B)
\[{{(N{{H}_{4}})}_{2}}Mn{{O}_{4}}\]
done
clear
C)
\[N{{H}_{4}}{{(Mn{{O}_{4}})}_{2}}\]
done
clear
D)
\[N{{H}_{4}}M{{n}_{2}}{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 73) Heart patients can be given
A)
monosaturated fatty acid
done
clear
B)
trans fatty acid
done
clear
C)
polyunsaturated fatty acid
done
clear
D)
polysaturated fatty acid
done
clear
View Answer play_arrow
question_answer 74) For the reaction 1 g mole of \[CaC{{O}_{3}}\] is enclosed in 5 L container \[CaC{{O}_{3}}(s)\xrightarrow{{}}CaO(s)+C{{O}_{2}}(g)\] \[{{k}_{p}}=11.6\] at 1073 K then per cent dissociation of \[CaC{{O}_{3}}\] is
A)
65%
done
clear
B)
100%
done
clear
C)
6.5%
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 75) The only amino acid which is non chiral is
A)
lysine
done
clear
B)
proline
done
clear
C)
glycine
done
clear
D)
histidine
done
clear
View Answer play_arrow
question_answer 76) The electrolyte \[[Co{{(N{{H}_{3}})}_{5}}Cl]C{{l}_{2}}\] is which of the following type?
A)
1 : 2
done
clear
B)
1 : 3
done
clear
C)
2 : 3
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 77) In heterogeneous catalysis which of the following process takes place?
A)
absorption
done
clear
B)
hydration
done
clear
C)
adsorption
done
clear
D)
intermediate compound
done
clear
View Answer play_arrow
question_answer 78) An ideal solution is obtained by mixing \[C{{H}_{3}}CHB{{r}_{2}}\] and \[C{{H}_{2}}Br-C{{H}_{2}}Br\] in the ratio \[2:1\,{{P}^{o}}_{C{{H}_{3}}CHB{{r}_{2}}}=173,\,{{P}^{o}}_{C{{H}_{2}}BrC{{H}_{2}}Br}=127\]
A)
158
done
clear
B)
257
done
clear
C)
137
done
clear
D)
197
done
clear
View Answer play_arrow
question_answer 79) For the reaction \[{{N}_{2}}+3{{H}_{2}}\rightleftarrows 2N{{H}_{3}}+\]heat when temperature increases cone. of
A)
formation of \[N{{H}_{3}}\] increases
done
clear
B)
formation of \[N{{H}_{3}}\] decreases
done
clear
C)
concentration of \[{{N}_{2}}\] decreases
done
clear
D)
concentration of \[{{H}_{2}}\] decreases
done
clear
View Answer play_arrow
question_answer 80) Carbon-oxygen bond length of \[C{{H}_{2}}O(I);C{{H}_{2}}\overset{-}{\mathop{O}}\,(II)\] and \[C{{H}_{3}}OH(III)\] is in the order
A)
\[I>II>III\]
done
clear
B)
\[II>I>III\]
done
clear
C)
\[III>II>I\]
done
clear
D)
\[III>I>II\]
done
clear
View Answer play_arrow
question_answer 81)
In the table Proton Electron Charge 23 X +3 46 43 Y
X and Y are
A)
20, -3
done
clear
B)
20, +3
done
clear
C)
26,-3
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 82) Which of the following is true for polypropylene?
A)
Propylene, condensation polymer
done
clear
B)
Propylene, addition polymer
done
clear
C)
Propylene, ammonic polymers
done
clear
D)
Propylene, cationic ammonic polymerase
done
clear
View Answer play_arrow
question_answer 83) The total number of orbital up to \[n=4\] are
A)
2
done
clear
B)
16
done
clear
C)
32
done
clear
D)
30
done
clear
View Answer play_arrow
question_answer 84) Coordination number of a body centered cubic is
A)
6
done
clear
B)
8
done
clear
C)
10
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 85) \[x\] mole of \[BaC{{O}_{3}}\] are soluble in 1 L then x is related to its solubility product by the expression
A)
\[x={{K}_{sp}}\]
done
clear
B)
\[{{x}^{2}}=\sqrt{{{K}_{sp}}}\]
done
clear
C)
\[x=\sqrt{{{K}_{sp}}}\]
done
clear
D)
\[x=\frac{{{K}_{sp}}}{2}\]
done
clear
View Answer play_arrow
question_answer 86) Which of the following do not have \[s{{p}^{2}}\]hybridised carbon?
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}OH\]
done
clear
C)
\[HCOOH\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 87) Isopiglmic solution are those which have
A)
same vapour pressure
done
clear
B)
same concentration
done
clear
C)
same density
done
clear
D)
same osmotic pressure
done
clear
View Answer play_arrow
question_answer 88) Which of the following is superoxide?
A)
\[N{{a}_{2}}{{O}_{2}}\]
done
clear
B)
\[K{{O}_{2}}\]
done
clear
C)
\[{{K}_{2}}O\]
done
clear
D)
\[{{C}_{3}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 89) The total number of isomers in \[{{C}_{6}}{{H}_{3}}C{{l}_{3}}\]
A)
two
done
clear
B)
three
done
clear
C)
four
done
clear
D)
five
done
clear
View Answer play_arrow
question_answer 90) In the reaction of \[NaH\]with water which of the following is present?
A)
\[Na{{O}^{-}}\]
done
clear
B)
\[O{{H}^{-}}\]
done
clear
C)
\[{{H}^{+}}\]
done
clear
D)
\[N{{a}^{-}}\]
done
clear
View Answer play_arrow
question_answer 91) \[NaH\] contains how many covalent bonds?
A)
zero
done
clear
B)
two
done
clear
C)
one
done
clear
D)
three
done
clear
View Answer play_arrow
question_answer 92) \[{{S}_{g}}\] and \[{{S}_{x}}\] are
A)
isotopes
done
clear
B)
isotones
done
clear
C)
allotropes
done
clear
D)
isobars
done
clear
View Answer play_arrow
question_answer 93) The total number of lone pair of \[Cl{{F}_{3}}\] are
A)
2
done
clear
B)
9
done
clear
C)
11
done
clear
D)
15
done
clear
View Answer play_arrow
question_answer 94) For the reaction between \[KMn{{O}_{4}}\] and \[{{H}_{2}}{{O}_{2}}\]the number of electrons transferred per mol of \[{{H}_{2}}{{O}_{2}}\] are
A)
one
done
clear
B)
two
done
clear
C)
three
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 95) What will be the resultant pH when 200 mL of an aqueous solution of \[HCl\,(pH=2)\] is mixed with 300 mL of an aqueous solution of\[NaOH\,(pH=12)\]?
A)
10
done
clear
B)
11
done
clear
C)
12
done
clear
D)
14
done
clear
View Answer play_arrow
question_answer 96) In the reaction \[10_{3}^{-}+S{{O}_{2}}+4{{H}_{2}}O\xrightarrow{{}}{{I}_{2}}+SO_{4}^{2-}+8{{H}^{+}}\] The coefficient of \[S{{O}_{2}}\] is
A)
three
done
clear
B)
four
done
clear
C)
five
done
clear
D)
six
done
clear
View Answer play_arrow
question_answer 97) Dilution of acid can be done by
A)
adding water to acid
done
clear
B)
adding acid to water
done
clear
C)
adding acid to minimum amount of water and then adding excess water
done
clear
D)
adding steam to acid
done
clear
View Answer play_arrow
question_answer 98) 1 mol/L of \[{{A}_{2}}\] is present and 11% get dissociated according to the equation\[{{A}_{2}}\rightleftarrows B+C\]\[{{K}_{c}}\] for the reaction is
A)
1.24
done
clear
B)
1.36
done
clear
C)
1.66
done
clear
D)
1.76
done
clear
View Answer play_arrow
question_answer 99) For the reaction \[2HX\xrightarrow{{}}{{H}_{2}}+{{X}_{2}}\] \[-\frac{d[HX]}{dt}=\]rate
A)
rate wrt \[HX=+\frac{1}{2}\frac{d[HX]}{dt}\]
done
clear
B)
rate wrt \[HX=-\frac{1}{2}\frac{d[HX]}{dt}\]
done
clear
C)
rate wrt \[HX=+\frac{d[HX]}{dt}\]
done
clear
D)
rate wrt \[HX=-\frac{d[HX]}{dt}\]
done
clear
View Answer play_arrow
question_answer 100) For the reaction \[_{6}^{11}C\xrightarrow{{}}_{5}^{11}B+X\] This is the example of
A)
positron emission
done
clear
B)
electron capture
done
clear
C)
electron emission
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 101) Resolving power of scanning electron microscope is
A)
0.001 nm
done
clear
B)
0.01 nm
done
clear
C)
20 nm
done
clear
D)
0.0001
done
clear
View Answer play_arrow
question_answer 102) Which of the following enzyme is used in making detergent?
A)
Amylase
done
clear
B)
Cellulase
done
clear
C)
Protease
done
clear
D)
Peptidase
done
clear
View Answer play_arrow
question_answer 103) Enzyme found functional in lysosome is
A)
acid phosphatase
done
clear
B)
basic phosphatase
done
clear
C)
oxido reductase
done
clear
D)
liases
done
clear
View Answer play_arrow
question_answer 104) Action of lysozyme is
A)
physiological
done
clear
B)
anatomical
done
clear
C)
morphological
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 105) Which is not phagocytic?
A)
Monocyte
done
clear
B)
Lymphocyte
done
clear
C)
Mast-cell
done
clear
D)
Neutrophil
done
clear
View Answer play_arrow
question_answer 106) Which part of brain control intellectual ability?
A)
Frontal lobe
done
clear
B)
Parietal lobe
done
clear
C)
Temporal lobe
done
clear
D)
Occipetal lobe
done
clear
View Answer play_arrow
question_answer 107) The correct sequence of meninges of brain from outside to inside is
A)
duramater \[\to \] arachnoid \[\to \] piamater
done
clear
B)
arachnoid \[\to \] duramater \[\to \] piamater
done
clear
C)
piamater \[\to \] duramater \[\to \]arachnoid
done
clear
D)
duramater \[\to \] piamater \[\to \] arachnoid
done
clear
View Answer play_arrow
question_answer 108) The sequence of ear ossicles from outside to inside is
A)
malleus \[\to \] incus \[\to \] stapes
done
clear
B)
incus \[\to \]stapes \[\to \] malleus
done
clear
C)
stapes \[\to \] incus \[\to \]malleus
done
clear
D)
malleus \[\to \]stapes \[\to \] incus
done
clear
View Answer play_arrow
question_answer 109) Which of the following is balancing organ?
A)
Organ of Corti
done
clear
B)
Cochlea
done
clear
C)
Vestibular region
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 110) Glenoid cavity is found in
A)
pelvic girdle
done
clear
B)
pectoral girdle
done
clear
C)
sternum
done
clear
D)
humerus
done
clear
View Answer play_arrow
question_answer 111) Vital capacity of lung is
A)
\[TV+IRV+ERV\]
done
clear
B)
\[TV+\text{ }IRV+\text{ }RV\]
done
clear
C)
\[TV+ERV\]
done
clear
D)
\[IRV+ERV\]
done
clear
View Answer play_arrow
question_answer 112) During normal respiration without any effort the volume of air inspired or expired is called
A)
tidal volume
done
clear
B)
reserve volume
done
clear
C)
residual volume
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 113) Synaptic vesicle is found in
A)
pre synaptic neuron
done
clear
B)
post synaptic neuron
done
clear
C)
synaptic cleft.
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 114) 3-PGA is first stable product ill
A)
carbon-reduction cycle
done
clear
B)
OAA
done
clear
C)
malic acid
done
clear
D)
PEP
done
clear
View Answer play_arrow
question_answer 115) Pat storing granules are
A)
elaioplast
done
clear
B)
amyloplast
done
clear
C)
aleuroplast
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 116) Sub units in prokaryotic ribosomcs are
A)
60S-40S
done
clear
B)
50S-30S
done
clear
C)
40S-30S
done
clear
D)
50S-20S
done
clear
View Answer play_arrow
question_answer 117) Adaptive radiation is for
A)
dissimilar character for adoption from common ancestor
done
clear
B)
similar characters for adoption from common ancestor
done
clear
C)
dissimilar character for adoption from different ancestor
done
clear
D)
similar characters for adoption from different ancestor
done
clear
View Answer play_arrow
question_answer 118) Competition of species leads to
A)
extinction
done
clear
B)
mutation
done
clear
C)
greater number of niches are formed
done
clear
D)
symbiosis
done
clear
View Answer play_arrow
question_answer 119) Large unit of land having different types of plants and animals
A)
uniform vegetation
done
clear
B)
biome
done
clear
C)
ecosystem
done
clear
D)
niche
done
clear
View Answer play_arrow
question_answer 120) 500 mL of 0.1 mol \[HCl\] has pH
A)
1
done
clear
B)
13
done
clear
C)
2
done
clear
D)
0.1
done
clear
View Answer play_arrow
question_answer 121) Pepsinogen is secreted by
A)
chief cell
done
clear
B)
1 oxyntic cell
done
clear
C)
mast cell
done
clear
D)
parietal cell
done
clear
View Answer play_arrow
question_answer 122) Haematuria means.
A)
RBC in the urine
done
clear
B)
WBC in the urine
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 123) Which of the following is both osmoregulator as well as nitrogenous product?
A)
\[N{{H}_{3}}\]
done
clear
B)
Urea
done
clear
C)
Uric acid
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 124) Phylogenetic system was given by
A)
Engler and Prantl
done
clear
B)
Pliny
done
clear
C)
John Ray
done
clear
D)
R.H. Whittaker
done
clear
View Answer play_arrow
question_answer 125) Coacervates are
A)
protobionts having polysaccharide + protein \[+{{H}_{2}}O\]
done
clear
B)
protein aggregate
done
clear
C)
protein and lipid aggregates
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 126) Bt toxin obtained from
A)
prokaryotes
done
clear
B)
eukaryotes
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 127) Which is correct?
A)
Slime moulds are haploid
done
clear
B)
Protozoan lack cell wall
done
clear
C)
Dinoflagellates are immotile
done
clear
D)
Pellicle is absent in Euglena
done
clear
View Answer play_arrow
question_answer 128) Zygospore is
A)
give rise to zoospores on meiosis
done
clear
B)
equivalent to Ascus, Brasilia
done
clear
C)
dormant stage
done
clear
D)
give raise to asexual spore
done
clear
View Answer play_arrow
question_answer 129) Mycorrhiza is found in
A)
oligotrophic soil
done
clear
B)
eutrophic soil
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 130) Which disaccharide has different linkage?
A)
Maltose
done
clear
B)
Starch
done
clear
C)
Sucrose
done
clear
D)
Lactose
done
clear
View Answer play_arrow
question_answer 131) Corpus luteum secretes
A)
progesterone and estrogen
done
clear
B)
LH
done
clear
C)
only progesterone
done
clear
D)
progesterone and LH
done
clear
View Answer play_arrow
question_answer 132) Testosterone is secreted by
A)
Leydig cell
done
clear
B)
Sertolicell
done
clear
C)
spermatogenic cell
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 133) Hypothyroidism causes
A)
myxodema
done
clear
B)
cretinism
done
clear
C)
both [a] and [b]
done
clear
D)
exopthalmic goiter
done
clear
View Answer play_arrow
question_answer 134) Which of the following controls the function of Sertoli cell?
A)
FSH
done
clear
B)
Estrogen
done
clear
C)
ACTH
done
clear
D)
Testosterone
done
clear
View Answer play_arrow
question_answer 135) Fertilization of sperm and ova takes place in
A)
ampulla of oviduct
done
clear
B)
isthmus of oviduct
done
clear
C)
fimbrae of oviduct
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 136) Rr rr progeny: Red (dominant) flowered heterozygous crossed with white flower
A)
350 \[\to \] red : 350 \[\to \] white
done
clear
B)
450 \[\to \] red : 250 white
done
clear
C)
380 \[\to \] red : 320 white
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 137) Linkage group in E. coli is/are
A)
4
done
clear
B)
2
done
clear
C)
1
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 138) Which of the following first suggest the conservative model of DNA replication?
A)
Cairns
done
clear
B)
Meselson and Stahl
done
clear
C)
Watson and Crick
done
clear
D)
Taylor
done
clear
View Answer play_arrow
question_answer 139) Lactic acid is formed in
A)
fermentation
done
clear
B)
glycolysis
done
clear
C)
HMP pathways
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 140) Red list in India completed by
A)
botanical survey of India
done
clear
B)
zoological survey of India
done
clear
C)
geological survey of India
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 141) National Botanical Institute is situated at
A)
Lucknow
done
clear
B)
Kolkotta
done
clear
C)
Mumbai
done
clear
D)
Chennai
done
clear
View Answer play_arrow
question_answer 142) Penicillin was used in
A)
I world war
done
clear
B)
II world war
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 143) Blindness prevented by use of which crop in poor country?
A)
golden rice
done
clear
B)
wheat
done
clear
C)
gram
done
clear
D)
pea
done
clear
View Answer play_arrow
question_answer 144) Ferula aesfoetida is obtain from
A)
roots and stem
done
clear
B)
leaves
done
clear
C)
stem
done
clear
D)
flower
done
clear
View Answer play_arrow
question_answer 145) Movement of \[{{H}_{2}}O\] through cell wall is
A)
apoplast
done
clear
B)
symplast
done
clear
C)
tonoplast
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 146) In which of the following animals Hb is found dissolved in plasma?
A)
Earthworm
done
clear
B)
Cockroach
done
clear
C)
5epia
done
clear
D)
Planaria
done
clear
View Answer play_arrow
question_answer 147) RAAS secretes which of the following hormone?
A)
mineralo corticoids
done
clear
B)
gluco corticoids
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 148) Bones become fragile in
A)
osteoporosis
done
clear
B)
gout
done
clear
C)
arthritis
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 149) Maximum percentage of lipoprotein is in:
A)
chylomicron
done
clear
B)
HDL
done
clear
C)
arthritis
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 150) During repolarization of nerve
A)
\[{{K}^{+}}\] gate closed and \[N{{a}^{+}}\] gate open
done
clear
B)
\[N{{a}^{+}}\] channels are closed and \[{{K}^{+}}\] channels are open
done
clear
C)
both gates remain open
done
clear
D)
both K+ and Na+ gates are closed
done
clear
View Answer play_arrow
question_answer 151) Zn, Mo, Fe, Cu are
A)
trace element
done
clear
B)
non-essential
done
clear
C)
macro nutrient
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 152) In haemoglobin which amino acid acts as blood buffer?
A)
histidine
done
clear
B)
glutamine
done
clear
C)
aspartic
done
clear
D)
lysine
done
clear
View Answer play_arrow
question_answer 153) Depict the correct site of hormone.
A)
\[\alpha \]-glucagons, \[\beta \]-insulin, \[\delta \]-somatostatin
done
clear
B)
\[\alpha \]-insulin, \[\beta \]-glucagons, \[\delta \]-somatostatin
done
clear
C)
\[\delta \]-insulin, \[\alpha \]-somatostatin, \[\beta \]-glucagons
done
clear
D)
\[\alpha \]-somatostadn, \[\beta \]-insulin, \[\delta \]-glucagons
done
clear
View Answer play_arrow
question_answer 154) Insulin receptors are
A)
extrinsic protein
done
clear
B)
intrinsic protein
done
clear
C)
G-protein
done
clear
D)
trimeric protein
done
clear
View Answer play_arrow
question_answer 155) Mode of feeding in free living protozoan is
A)
holozoic
done
clear
B)
saprozoic
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 156) Protein deficiency leads to
A)
kwashiorkor
done
clear
B)
marasmus
done
clear
C)
cretinism
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 157) Chloride shift in respond to
A)
\[HCO_{3}^{-}\]
done
clear
B)
\[{{K}^{+}}\]
done
clear
C)
\[{{H}^{+}}\]
done
clear
D)
\[N{{a}^{+}}\]
done
clear
View Answer play_arrow
question_answer 158) Double fertilization involves
A)
syngamy + triple fusion .
done
clear
B)
double fertilization
done
clear
C)
development of antipodal colls
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 159) Late blight of potato is caused by
A)
Phytophothora infestans
done
clear
B)
Xanthomonas oryzae
done
clear
C)
Puccinia graminis
done
clear
D)
TMV
done
clear
View Answer play_arrow
question_answer 160) Tendril of Cucurbita and thorns of Bouganvellia are
A)
homologous organ
done
clear
B)
analogous organ
done
clear
C)
vestigial organ
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 161) \[{{O}_{2}}\] dissociation curve is
A)
sigmoid curve
done
clear
B)
parabolic
done
clear
C)
hyperbolic
done
clear
D)
straight line
done
clear
View Answer play_arrow
question_answer 162) IAA is derived from or which of the following is involved in the synthesis of a plant hormone IAA and Vasoconstrides cerotonin?
A)
tryptophan
done
clear
B)
tyrosine
done
clear
C)
phenylalamine
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 163) Transverse binary fission is found in
A)
Paramecium
done
clear
B)
Amoeba
done
clear
C)
Hydra
done
clear
D)
Euglena
done
clear
View Answer play_arrow
question_answer 164) Capacitation of sperm occurs in
A)
female genetical tract
done
clear
B)
vas defferens
done
clear
C)
vas efferens
done
clear
D)
vagina
done
clear
View Answer play_arrow
question_answer 165) Phytoplanktons are found in which of the following zone?
A)
limnetic zone
done
clear
B)
profundal zone
done
clear
C)
littoral
done
clear
D)
aphotic zone
done
clear
View Answer play_arrow
question_answer 166) Estuaries are considered of nutrient and trap
A)
river
done
clear
B)
pond
done
clear
C)
lake
done
clear
D)
ocean
done
clear
View Answer play_arrow
question_answer 167) Green algae contains
A)
chlorophyll [a] and [b]
done
clear
B)
starch
done
clear
C)
carotenoid
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 168) Branch of biology dealing with study of organism in outer space is
A)
Exobiology
done
clear
B)
Ethology
done
clear
C)
Euphenics
done
clear
D)
Ethnology
done
clear
View Answer play_arrow
question_answer 169) Each immunoglobin has two heavy chains and two light chains, the antigen binding is present in
A)
variable region of heavy chain
done
clear
B)
variable region of both heavy and light chain
done
clear
C)
variable region of light chain
done
clear
D)
constant region of both light and heavy chain
done
clear
View Answer play_arrow
question_answer 170) Extension of plasma membrane in prokaryotic cell is
A)
mesosome
done
clear
B)
hapnoid
done
clear
C)
ribosome
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 171) Central dogma of genetic information modified by the discovery of
A)
Reverse transcriptase
done
clear
B)
DNA polymerase
done
clear
C)
RNA polymerase
done
clear
D)
ligase
done
clear
View Answer play_arrow
question_answer 172) Incorrect character of brown algae is
A)
chl. a and b present
done
clear
B)
they remain attached
done
clear
C)
chl a and c present
done
clear
D)
presence of fucoxanthin
done
clear
View Answer play_arrow
question_answer 173) Digestive enzymes are
A)
hydrolases
done
clear
B)
oxido reductases
done
clear
C)
transferases
done
clear
D)
lyases
done
clear
View Answer play_arrow
question_answer 174) Number of proiofilament in microtubule is
A)
13
done
clear
B)
12
done
clear
C)
5
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 175) Which is not involved as 2nd messenger in \[C{{a}^{2+}}\] mediated hormone?
A)
c-AMP
done
clear
B)
DG
done
clear
C)
Phospholipase
done
clear
D)
\[I{{P}_{3}}\]
done
clear
View Answer play_arrow
question_answer 176) Protein found in eye lens is
A)
crystalline
done
clear
B)
collagen
done
clear
C)
opsin
done
clear
D)
rhodopsin
done
clear
View Answer play_arrow
question_answer 177) Which of the following has been covered under the broad patent category?
A)
Triticum
done
clear
B)
Oryza
done
clear
C)
Pissum sativum
done
clear
D)
Brassica
done
clear
View Answer play_arrow
question_answer 178) Alteration of which genes leads to cancer?
A)
cell proliferation gene (protooncogenes)
done
clear
B)
tumour suppressor gene
done
clear
C)
tumour virus gene
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 179) Which cell secretes antibody?
A)
Lymphocytes
done
clear
B)
Monocytes
done
clear
C)
Eosinophil
done
clear
D)
Neutrophil
done
clear
View Answer play_arrow
question_answer 180) Jojoba contains
A)
C-20 to C-6 bromohydric alcohol wax and triglyceride
done
clear
B)
wax
done
clear
C)
triglyceride
done
clear
D)
sterol
done
clear
View Answer play_arrow
question_answer 181) Turner syndrome is
A)
XO
done
clear
B)
XXY
done
clear
C)
XXX
done
clear
D)
XYY
done
clear
View Answer play_arrow
question_answer 182) Operon concept was proposed by
A)
Jacob and Monod
done
clear
B)
David Baltimore
done
clear
C)
Alice Jaffcry
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 183) Blood clotting vitamin is
A)
vitamin-K
done
clear
B)
vitamin-A
done
clear
C)
vitamin-\[{{B}_{12}}\]
done
clear
D)
vkamin-\[{{B}_{6}}\]
done
clear
View Answer play_arrow
question_answer 184) Expand ELISA
A)
enzyme linked immuno sorbent assay
done
clear
B)
enzyme linked ion sorbent assay
done
clear
C)
enzyme linked inductive assay
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 185) Internodes elongation is due to
A)
gibberellin
done
clear
B)
auxin
done
clear
C)
cytokinin
done
clear
D)
abscisic acid
done
clear
View Answer play_arrow
question_answer 186) Apical dominance is due to
A)
auxin
done
clear
B)
cytokinin
done
clear
C)
ethylene
done
clear
D)
gibberellins
done
clear
View Answer play_arrow
question_answer 187) Gibberellin causes
A)
apical dominance
done
clear
B)
flowering
done
clear
C)
interned al growth
done
clear
D)
wilting
done
clear
View Answer play_arrow
question_answer 188) If non-limiting condition are provided then, which happens?
A)
natality increase and mortality decreases
done
clear
B)
morality decrease
done
clear
C)
natality increases
done
clear
D)
motality increases
done
clear
View Answer play_arrow
question_answer 189) Mother B homozygous, father A unknown \[\therefore \] possible blood group in progeny is
A)
AB and B possible
done
clear
B)
AB and A possible
done
clear
C)
A + B possible
done
clear
D)
0 possible
done
clear
View Answer play_arrow
question_answer 190) In flagella membrane which enzyme catalyses ATP activity?
A)
cytoplasmic dyenin
done
clear
B)
asconic dynein
done
clear
C)
kinesis
done
clear
D)
myosin
done
clear
View Answer play_arrow
question_answer 191) The new varieties of plants are produced by
A)
selection and hybridization
done
clear
B)
mutation and selection
done
clear
C)
introduction and mutation
done
clear
D)
selection and introduction
done
clear
View Answer play_arrow
question_answer 192) Differentiated cell remains at which stage?
A)
\[{{G}_{1}}\]
done
clear
B)
\[{{G}_{2}}\]
done
clear
C)
\[{{G}_{0}}\]
done
clear
D)
M
done
clear
View Answer play_arrow
question_answer 193) Genetic material of retrovirus is
A)
DNA
done
clear
B)
RNA
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 194) Characters of acquired immunity are
A)
specificity
done
clear
B)
difference between self and non-self
done
clear
C)
retains memory
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 195) Which conserved motifs is found in E. coli genes?
A)
TATA box
done
clear
B)
CAAT box
done
clear
C)
Pribnow box
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 196) Vernalization is done at
A)
lower temperature
done
clear
B)
low light intensity
done
clear
C)
higher temperature
done
clear
D)
high light intensity
done
clear
View Answer play_arrow
question_answer 197) Which of the following is involved in the catalysis of link reaction during aerobic respiration?
A)
Vitamin-A
done
clear
B)
Vitamin-\[{{B}_{1}}\]
done
clear
C)
Vitamin-\[{{B}_{6}}\]
done
clear
D)
Vitamin-K
done
clear
View Answer play_arrow
question_answer 198) Archaeopteryx is a connecting link between
A)
Pisces and Amphibia
done
clear
B)
Reptiles and Aves
done
clear
C)
Amphibia and Aves
done
clear
D)
Reptiles and Mammals
done
clear
View Answer play_arrow
question_answer 199) A tall plant was grown in nutrient deficient soil and remained dwarf. When it is crossed with dwarf plant then
A)
all hybrid plants are dwarf
done
clear
B)
all hybrid plants are tall
done
clear
C)
50% tall and 50% dwarf
done
clear
D)
75% tall and 25% dwarf
done
clear
View Answer play_arrow
question_answer 200) Which one is correct sequence in glycolysis?
A)
G6-P \[\to \] PEP \[\to \] 3-PGAL \[\to \] 3PGA
done
clear
B)
G6-P \[\to \] 3-PGAL \[\to \] 3-PGA \[\to \] PEP
done
clear
C)
G6-P \[\to \] PEP \[\to \] 3-PGA \[\to \] 3-PGAL
done
clear
D)
G6-P \[\to \] 3-PGA \[\to \] 3-PGAL \[\to \] PEP
done
clear
View Answer play_arrow
 Concave mirror
Concave mirror

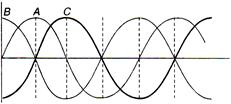 With respect to A, the progressive wave
With respect to A, the progressive wave
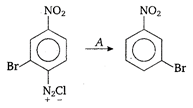 A is
A is