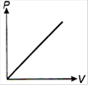
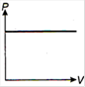
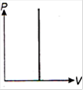
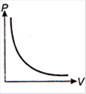
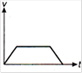
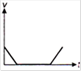
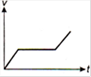
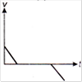
question_answer 1) Which of the following graphs between pressure and volume correctly shows isochoric change?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 2) In a thermodynamic process, pressure of a fixed mass of a gas is changed in such a manner that the gas molecules give out 30 J of heat and 10 J of work is done on the gas. If the initial internal energy of the gas was 40 J, then the final internal energy will be:
A)
30 J
done
clear
B)
20 J
done
clear
C)
60 J
done
clear
D)
40 J
done
clear
View Answer play_arrow
question_answer 3) The rms speed of a group of 7 gas molecules having speeds (6, 4, 2, 0, - 2, - 4, - 6) m/s is :
A)
1.5 m/s
done
clear
B)
3.4 m/s
done
clear
C)
9 m/s
done
clear
D)
4 m/s
done
clear
View Answer play_arrow
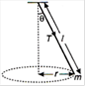
question_answer 4)
A string of length \[l\]fixed at one end carries a mass m at the other end. The string makes \[\frac{2}{\pi }\]rev/s around the horizontal axis through the fixed end as shown in the figure, the tension in string is :
A)
\[16\,ml\]
done
clear
B)
\[4\,ml\]
done
clear
C)
\[8\,ml\]
done
clear
D)
\[2\,ml\]
done
clear
View Answer play_arrow
question_answer 5) An exploding star is called :
A)
nova
done
clear
B)
supernova
done
clear
C)
black hole
done
clear
D)
neutron star
done
clear
View Answer play_arrow
question_answer 6) For a transistor, in a common emitter arrangement, the alternating current gain P is given by :
A)
\[\beta ={{\left( \frac{\Delta {{l}_{C}}}{\Delta {{l}_{B}}} \right)}_{{{V}_{CE}}}}\]
done
clear
B)
\[\beta ={{\left( \frac{\Delta {{l}_{B}}}{\Delta {{l}_{C}}} \right)}_{{{V}_{CE}}}}\]
done
clear
C)
\[\beta ={{\left( \frac{\Delta {{l}_{C}}}{\Delta {{l}_{E}}} \right)}_{{{V}_{CE}}}}\]
done
clear
D)
\[\beta ={{\left( \frac{\Delta {{l}_{E}}}{\Delta {{l}_{C}}} \right)}_{{{V}_{CE}}}}\]
done
clear
View Answer play_arrow
question_answer 7) A magnetic field :
A)
always exerts a force on charged particle
done
clear
B)
never exerts a force on charged particle
done
clear
C)
exerts a force, if the charged panicle is moving across the magnetic field lines
done
clear
D)
exerts a force, if the charged particle is moving along the magnetic field lines
done
clear
View Answer play_arrow
question_answer 8) The resistance of tungsten filament at \[{{150}^{o}}C\] is \[133\,\Omega \] what will be its resistance at \[{{500}^{o}}C\]? The temperature coefficient of resistance of tungsten is \[0.0045{{/}^{o}}C\].
A)
\[366\,\Omega \]
done
clear
B)
\[69\,\Omega \]
done
clear
C)
\[266\,\Omega \]
done
clear
D)
\[109\,\Omega \]
done
clear
View Answer play_arrow
question_answer 9) The dimensional formula for the coefficient of viscosity is :
A)
\[[M{{L}^{-1}}{{T}^{-1}}\text{ }\!\!]\!\!\text{ }\]
done
clear
B)
\[[M{{L}^{-1}}{{T}^{-3}}\text{ }\!\!]\!\!\text{ }\]
done
clear
C)
\[[M{{L}^{-2}}{{T}^{-2}}\text{ }\!\!]\!\!\text{ }\]
done
clear
D)
\[[M{{L}^{2}}{{T}^{-2}}\text{ }\!\!]\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 10) The half-life of radium is 1600 yr. The fraction of a sample of radium that would remain after 6400 yr is :
A)
1/4
done
clear
B)
1/2
done
clear
C)
1/8
done
clear
D)
1/16
done
clear
View Answer play_arrow
question_answer 11) If the wavelength of 1st line of Balmer series of hydrogen is \[6561\,\overset{o}{\mathop{A}}\,\], the wavelength of the 2nd line of series will be :
A)
\[\text{9780}\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[\text{48660}\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[\text{8857}\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[\text{4429}\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 12) If the energy of a photon corresponding to a wavelength of \[6000\,\overset{o}{\mathop{A}}\,\]is\[3.32\times {{10}^{-19}}J\], the photon energy for a wavelength of \[\text{4000}\,\overset{\text{o}}{\mathop{\text{A}}}\,\] will be:
A)
1.4 eV
done
clear
B)
4.9 eV
done
clear
C)
3.1 eV
done
clear
D)
1.6 eV
done
clear
View Answer play_arrow
question_answer 13) The probability of electrons to be found in the conduction band of an intrinsic semiconductor at a finite temperature :
A)
decreases exponentially with increasing band gap
done
clear
B)
increases exponentially with increasing band gap
done
clear
C)
decreases with increasing temperature
done
clear
D)
is independent of the temperature and the band gap
done
clear
View Answer play_arrow
question_answer 14) The first overtone of a stretched wire of given length is 320 Hz. The first harmonic is :
A)
320 Hz
done
clear
B)
160 Hz
done
clear
C)
480 Hz
done
clear
D)
640 Hz
done
clear
View Answer play_arrow
question_answer 15) What is minimum length of a tube, open at both ends, that resonates with tuning fork of frequency 350 Hz? (velocity of sound in air = 350 m/s]
A)
50 cm
done
clear
B)
100 cm
done
clear
C)
75cm
done
clear
D)
25cm
done
clear
View Answer play_arrow
question_answer 16) The magnifying power of a telescope is 9. When it is adjusted for parallel rays, the distance between the objective and the eyepiece is found to be 20 cm. The focal lengths of lenses are:
A)
18 cm, 2 cm
done
clear
B)
11 cm, 9 cm
done
clear
C)
10 cm, 10 cm
done
clear
D)
15 cm, 5 cm
done
clear
View Answer play_arrow
question_answer 17) In Youngs double slit experiment, the fringe width is found to be 0.4 mm. If the whole apparatus is immersed in water of refractive index 4/3, without disturbing the geometrical arrangement, the new fringe width will be :
A)
0.30mm
done
clear
B)
0.40mm
done
clear
C)
0.53mm
done
clear
D)
450 urn
done
clear
View Answer play_arrow
question_answer 18) A substance breaks down by a stress of\[{{10}^{6}}N/{{m}^{2}}\]. If the density of the material of the wire is \[3\times {{10}^{3}}kg/{{m}^{3}}\], then the length of the wire of the substance which will break under its own weight when suspended vertically, is:
A)
66.6m
done
clear
B)
60.0m
done
clear
C)
33,3m
done
clear
D)
30.0m
done
clear
View Answer play_arrow
question_answer 19) A square wire frame of size L is dipped in a liquid. On taking out, a membrane is formed. If the surface tension of liquid is T, the force acting on the frame will be :
A)
2TL
done
clear
B)
4TL
done
clear
C)
8TL
done
clear
D)
10 TL
done
clear
View Answer play_arrow
question_answer 20) A black body radiates energy at the rate of \[1\times {{10}^{5}}J/s{{m}^{2}}\] at a temperature of \[{{227}^{o}}C\]. The temperature to which it must be heated, so that it radiates energy at the rate of \[1\times {{10}^{9}}J/s{{m}^{2}}\], is:
A)
5000 K
done
clear
B)
\[{{5000}^{o}}C\]
done
clear
C)
500 K
done
clear
D)
\[{{500}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 21) A stretched string is vibrating according to the equation \[y=5\,\sin \,\left( \frac{\pi x}{2} \right)\cos \,4\pi t\] where \[y\] and \[x\] are in cm and t in second. The distance between two consecutive nodes on the string is:
A)
2 cm
done
clear
B)
4 cm
done
clear
C)
8 cm
done
clear
D)
16 cm
done
clear
View Answer play_arrow
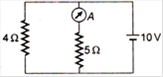
question_answer 22)
In the given diagram, the reading of the ammeter is (when the internal resistance of the battery is zero) :
A)
40/29 A
done
clear
B)
10/9 A
done
clear
C)
5/3 A
done
clear
D)
2 A
done
clear
View Answer play_arrow
question_answer 23) Photons of energy 5.5eV fall on the surface of the metal emitting photoelectrons of maximum kinetic energy 4.0eV. The stopping potential required for these electrons are :
A)
5.5V
done
clear
B)
1.5V
done
clear
C)
9.5V
done
clear
D)
4.0V
done
clear
View Answer play_arrow
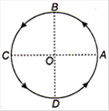
question_answer 24)
Figure shows a body of mass m moving with a uniform speed v along a circle of radius r. The change in velocity in going from A to B is:
A)
\[v\sqrt{2}\]
done
clear
B)
\[v/\sqrt{2}\]
done
clear
C)
\[v\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 25) Which radiations are used in treatment of muscles ache?
A)
Infrared
done
clear
B)
Ultraviolet
done
clear
C)
Microwave
done
clear
D)
X-rays
done
clear
View Answer play_arrow
question_answer 26) Angular width \[(\theta )\] of central maximum of a diffraction pattern of a single slit does not depend upon:
A)
distance between slit and source
done
clear
B)
wavelength of light used
done
clear
C)
width of the slit
done
clear
D)
frequency of light used
done
clear
View Answer play_arrow
question_answer 27) A 15 g ball is shot from a spring gun whose spring has a force constant of 600 N/m. The spring is compressed by 5 cm. The greatest possible horizontal range of the ball for this compression is: \[(g=10\,m/{{s}^{2}})\]
A)
6.0m
done
clear
B)
10.0m
done
clear
C)
12.0m
done
clear
D)
8.0m
done
clear
View Answer play_arrow
question_answer 28) An engine pumps up 100 kg of water trough a height of 10 m in 5 s. Given that the efficiency of the engine is 60%. If \[g=10\,m{{s}^{-2}}\], the power of the engine is :
A)
3.3 kW
done
clear
B)
0.33 kW
done
clear
C)
0.033 kW
done
clear
D)
33 kW
done
clear
View Answer play_arrow
question_answer 29) The conduction current is same as displacement current when source is :
A)
AC only
done
clear
B)
DC only
done
clear
C)
both AC and DC
done
clear
D)
neither DC nor AC
done
clear
View Answer play_arrow
question_answer 30) A transformer is having 2100 turns in primary and 4200 turns in secondary. An AC source of 120 V, 10 A is connected to its primary. The secondary voltage and current are :
A)
240 V, 5 A
done
clear
B)
120 V, 10 A
done
clear
C)
240 V, 10 A
done
clear
D)
120 V, 20 A
done
clear
View Answer play_arrow
question_answer 31) A rectangular coil of 20 turns and area of cross section \[25\,\,c{{m}^{2}}\] has a resistance of \[100\,\,\Omega \]. If a magnetic Held which is perpendicular to the plane of coil changes at a rate of 1000 T/s, the current in the coil is :
A)
1A
done
clear
B)
50 A
done
clear
C)
0.5 A
done
clear
D)
5 A
done
clear
View Answer play_arrow
question_answer 32) A vertical straight conductor carries a current vertically upwards. A point P lies to the east of it at a small distance and another point Q lies to the west at the same distance. The magnetic field at P is :
A)
greater than at Q
done
clear
B)
same as at Q
done
clear
C)
less than at Q
done
clear
D)
greater or less than at Q, depending upon the strength of current
done
clear
View Answer play_arrow
question_answer 33) A voltmeter has resistance of G ohm and range of V volt. The value of resistance used in series to convert it into a voltmeter of range nV volt is:
A)
\[n\,\,\,G\]
done
clear
B)
\[(n-1)\,\,G\]
done
clear
C)
\[\frac{G}{n}\]
done
clear
D)
\[\frac{G}{n-1}\]
done
clear
View Answer play_arrow
question_answer 34)
A bar magnet of length 3 cm has points
A)
8
done
clear
B)
\[1/2\sqrt{2}\]
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 35) A particle in SHM is described by the displacement equation \[x\,(t)=A\,\cos \,(\omega \,t+\theta )\]. If the initial \[(t=0)\] position of the particle is 1 cm and its initial velocity is \[\pi \,cm/s\], what is its amplitude? The angular frequency of the particle is \[\pi {{s}^{-1}}:\]
A)
1cm
done
clear
B)
\[\sqrt{2}\]cm
done
clear
C)
2cm
done
clear
D)
2.5cm
done
clear
View Answer play_arrow
question_answer 36) The magnetic Held at a distance r from a long wire carrying current i is 0.4 T. The magnetic field at a distance 2 r is :
A)
0.2T
done
clear
B)
0.8 T
done
clear
C)
0.1 T
done
clear
D)
1.6 T
done
clear
View Answer play_arrow
question_answer 37) An underwater sonar source operating at a frequency of 60 kHz directs its beam towards the surface. If velocity of sound in air is 330 m/s, wavelength and frequency of the waves in air are :
A)
5.5 mm, 60 kHz
done
clear
B)
330 m, 60 kHz
done
clear
C)
5.5 mm, 30 kHz
done
clear
D)
5.5 mm, 80 kHz
done
clear
View Answer play_arrow
question_answer 38) Velocity of light in diamond, glass and water decreases in the following order :
A)
water > glass > diamond
done
clear
B)
diamond > glass > water
done
clear
C)
diamond > water > glass
done
clear
D)
water > diamond > glass
done
clear
View Answer play_arrow
question_answer 39) A child swinging on a swing m a sitting position, stands up, then the time period of the swing will:
A)
increase
done
clear
B)
decrease
done
clear
C)
remain same
done
clear
D)
increase if the child is long and decrease if the child is short
done
clear
View Answer play_arrow
question_answer 40) When a sphere of moment of inertia \[I\]about an axis through centre of gravity and mass m rolls from rest down an inclined plane without slipping, its kinetic energy is :
A)
\[\frac{1}{2}I{{\omega }^{2}}\]
done
clear
B)
\[\frac{1}{2}m{{v}^{2}}\]
done
clear
C)
\[I\omega +mv\]
done
clear
D)
\[\frac{1}{2}I{{\omega }^{2}}+\frac{1}{2}m{{v}^{2}}\]
done
clear
View Answer play_arrow
question_answer 41) The angular momentum of a system of particles is not conserved :
A)
when a net external force acts upon the system
done
clear
B)
when a net external torque is acting upon the system
done
clear
C)
when a net external impulse is acting upon the system
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 42) A truck and a car are moving with equal velocity. On applying brakes, both will stop after certain distance, then :
A)
truck will cover less distance before stopping
done
clear
B)
car will cover less distance before stopping
done
clear
C)
both will cover equal distance
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 43) A lift is moving downwards with an acceleration equal to acceleration due to gravity. A body of mass m kept on the floor of the lift is pulled horizontally. If the coefficient of friction is \[\mu \] then the frictional resistance offered by the body is :
A)
\[mg\]
done
clear
B)
\[\mu mg\]
done
clear
C)
\[2\mu mg\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 44) A ball is projected upwards from the top of tower with a velocity \[50\,\,m{{s}^{-1}}\] making an angle \[{{30}^{o}}\] with the horizontal. The height of tower is 70 m. After how many seconds from the instant of throwing will the ball reach the ground?
A)
2s
done
clear
B)
5s
done
clear
C)
7s
done
clear
D)
9s
done
clear
View Answer play_arrow
question_answer 45) Find the torque of a force \[\vec{F}=-3\hat{i}+\hat{j}+5\hat{k}\]acting at the point \[\vec{r}=7\hat{i}+3\,\hat{J}+\hat{k}\]
A)
\[14\hat{i}-38\,\hat{J}+16\,\hat{k}\]
done
clear
B)
\[4\hat{i}+4\,\hat{J}+6\,\hat{k}\]
done
clear
C)
\[-14\hat{i}+38\,\hat{J}-16\,\hat{k}\]
done
clear
D)
\[-21\,\hat{i}+3\,\hat{J}+5\,\hat{k}\]
done
clear
View Answer play_arrow
question_answer 46) A stone released with zero velocity from the top of tower reaches the ground in 4 s. The height of the tower is about:
A)
20m
done
clear
B)
40m
done
clear
C)
80m
done
clear
D)
160m
done
clear
View Answer play_arrow
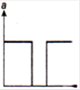
question_answer 47)
Acceleration-time graph of a body is shown. The Corresponding velocity-time graph of the same body is:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 48) A hollow metal sphere of radius 5 cm is charged such that the potential on its surface is10 V. The potential at a distance of 2 cm from the center of the sphere is :
A)
zero
done
clear
B)
10 V
done
clear
C)
4V
done
clear
D)
10/3V
done
clear
View Answer play_arrow
question_answer 49) In a certain charge distribution, all points having zero potential can be joined by a circle an S. Points inside S have positive potential points outside S have negative potential. A positive charge, which is free to move, is placed inside S:
A)
it will remain in equilibrium
done
clear
B)
it can move inside S, but it cannot cross S
done
clear
C)
it must cross S at some time
done
clear
D)
it may move, but will ultimately return to its starting point
done
clear
View Answer play_arrow
question_answer 50) The escape velocity from earth is\[11.2\,\,km\,{{s}^{-1}}\]. Another planet is having mass 1000 times and radius 10 times that of the earth, then escape velocity at that planet will be:
A)
11.2 km/s
done
clear
B)
112 km/s
done
clear
C)
1.12 km/s
done
clear
D)
1120 km/s
done
clear
View Answer play_arrow
question_answer 51) In Friedel-Crafts acylation, besides \[AlC{{l}_{3}}\], the other reactants are :
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 52) The prefix zepto stands for:
A)
\[{{10}^{9}}\]
done
clear
B)
\[{{10}^{-12}}\]
done
clear
C)
\[{{10}^{-15}}\]
done
clear
D)
\[{{10}^{-21}}\]
done
clear
View Answer play_arrow
question_answer 53) The boiling point of benzene is 353.23 K. When 1.80 g of a nonvolatile solute was dissolved in 90 g of benzene, the boiling point is raised to 354.11 K. The molar mass of the solute is : [\[{{K}_{b}}\] for benzene = 2.53 K kg \[mo{{l}^{-1}}\]]
A)
5.8 g \[mo{{l}^{-1}}\]
done
clear
B)
0.58 g \[mo{{l}^{-1}}\]
done
clear
C)
58 g \[mo{{l}^{-1}}\]
done
clear
D)
0.88 g \[mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 54) One fermi is :
A)
\[{{10}^{-13}}cm\]
done
clear
B)
\[{{10}^{-15}}cm\]
done
clear
C)
\[{{10}^{-10}}cm\]
done
clear
D)
\[{{10}^{-12}}cm\]
done
clear
View Answer play_arrow
question_answer 55) Which of the following reagents will produce salicylaldehyde on reaction with phenol?
A)
\[CHC{{l}_{3}}/NaOH\]
done
clear
B)
\[CC{{l}_{4}}/NaOH\]
done
clear
C)
\[C{{H}_{2}}C{{l}_{2}}/NaOH\]
done
clear
D)
\[C{{H}_{3}}Cl/NaOH\]
done
clear
View Answer play_arrow
question_answer 56) Which of the following is correct?
A)
Crystal system - Cubic Axial Distance - \[a\ne b=c\] Axial angles - \[\alpha =\beta \ne \gamma \] Examples - Cu, KCl
done
clear
B)
Crystal system - Monoclinic Axial Distance - \[\alpha \ne b=c\]Axial angles - \[\alpha \ne \beta =\gamma \]\[={{90}^{o}}\]Examples - \[pbCr{{O}_{2}}\],\[pbCr{{O}_{4}}\]
done
clear
C)
Crystal system - Rhombohedral Axial Distance - a = b = c Axial angles - \[\alpha =\beta =\gamma \]\[\ne {{90}^{o}}\] Examples - \[CaC{{O}_{3}}\],Hgs
done
clear
D)
Crystal system - Triclinic Axial Distance - a = b = c Axial angles - \[\alpha \ne \beta =\gamma \]\[\ne {{90}^{o}}\] Examples - \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]\[CuS{{O}_{4}}5{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 57) The monomer of nylon-6 is/are :
A)
\[HO-C{{H}_{3}}-C{{H}_{2}}-OH\]
done
clear
B)
done
clear
C)
\[{{F}_{2}}C=C{{F}_{2}}\]
done
clear
D)
\[{{H}_{2}}C=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 58) The enthalpy change for the reaction of 50.00 mL of ethylene with 50.00 mL of H^ at 1.5 atm pressure is \[\Delta H=-0.31\,kJ\]. The value of \[\Delta E\]will be:
A)
0.235 kJ
done
clear
B)
0.3024 kJ
done
clear
C)
2.567 kJ
done
clear
D)
0.0076 kJ
done
clear
View Answer play_arrow
question_answer 59) The solubility product of \[A{{g}_{2}}Cr{{O}_{4}}\] is\[32\times {{10}^{-12}}\]. What is the concentration of \[CrO_{4}^{2-}\] ions in that solution (in g ions \[{{L}^{-1}}\])?
A)
\[2\times {{10}^{-4}}\]
done
clear
B)
\[8\times {{10}^{-4}}\]
done
clear
C)
\[8\times {{10}^{-8}}\]
done
clear
D)
\[16\times {{10}^{-4}}\]
done
clear
View Answer play_arrow
question_answer 60) On reduction with hydrogen, 3.6 g of an oxide of metal left 3.2 g of metal. If the vapour density of metal is 32 , the simplest formula of the oxide would be :
A)
MO
done
clear
B)
\[{{M}_{2}}{{O}_{3}}\]
done
clear
C)
\[{{M}_{2}}O\]
done
clear
D)
\[{{M}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 61) Lattice energy of alkali metal chlorides follows the order :
A)
\[LiCl>NaCl>KCl>RbCl>CsCl\]
done
clear
B)
\[CsCl>NaCl>KCl>RbCl>LiCl\]
done
clear
C)
\[LiCl>CsCl>NaCl>KCl>RbCl\]
done
clear
D)
\[NaCl>LiCl>KCl>RbCl>CsCl\]
done
clear
View Answer play_arrow
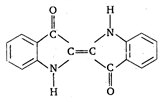
question_answer 62) The structural formula of indigo dye is :
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 63) Calculate \[\Delta {{G}^{o}}\] for conversion of oxygen? To ozone \[3/2{{O}_{2}}(g)\xrightarrow{{}}{{O}_{3}}(g)\]at 298 K, if \[{{K}_{p}}\]for this conversion is \[2.47\times {{10}^{-29}}\]:
A)
163 kJ \[mo{{l}^{-1}}\]
done
clear
B)
\[2.4\times {{10}^{2}}kJ\,mo{{l}^{-1}}\]
done
clear
C)
1.63 kJ \[mo{{l}^{-1}}\]
done
clear
D)
\[2.38\times {{10}^{6}}kJ\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 64) The right order of the solubility of sulphates of alkaline earth metals in water is :
A)
\[Be>Ca>Mg>Ba>Sr\]
done
clear
B)
\[Mg>Be>Ba>Ca>Sr\]
done
clear
C)
\[Be>Mg>Ca>Sr>Ba\]
done
clear
D)
\[Mg>Ca>Ba>Be>Sr\]
done
clear
View Answer play_arrow
question_answer 65) Gas A is bubbled through slaked lime when a white precipitate is formed. On prolonged bubbling, the precipitate is dissolved. On heating the resultant solution, the white precipitate reappears with the evolution of gas B. The gases A and B respectively are:
A)
\[C{{O}_{2}}\] and CO
done
clear
B)
CO and \[C{{O}_{2}}\]
done
clear
C)
CO and CO
done
clear
D)
CO 2 and \[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 66) Which of the following statements is true for fuel cells?
A)
They run till the reactants are active
done
clear
B)
They are free from pollution
done
clear
C)
They are more efficient
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 67) \[R-CH=C{{H}_{2}}+CO+{{H}_{2}}\]\[\xrightarrow[high\text{ }pressure]{High\,\,tem}RC{{H}_{2}}C{{H}_{2}}CHO\] The above reaction is:
A)
Mendius reaction
done
clear
B)
Oxo process
done
clear
C)
Sandmeyer reaction
done
clear
D)
Stephens reaction
done
clear
View Answer play_arrow
question_answer 68) Of the following acids, the one that is strongest is :
A)
\[HBr{{O}_{4}}\]
done
clear
B)
\[HOCl\]
done
clear
C)
\[HN{{O}_{2}}\]
done
clear
D)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 69) The boiling points of the following hydrides follow the order of:
A)
\[N{{H}_{3}}>As{{H}_{3}}>P{{H}_{3}}>Sb{{H}_{3}}\]
done
clear
B)
\[Sb{{H}_{3}}>As{{H}_{3}}>P{{H}_{3}}>N{{H}_{3}}\]
done
clear
C)
\[Sb{{H}_{3}}>N{{H}_{3}}>As{{H}_{3}}>P{{H}_{3}}\]
done
clear
D)
\[N{{H}_{3}}>P{{H}_{3}}>As{{H}_{3}}>Sb{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 70) Strongest conjugate base is :
A)
\[C{{l}^{-}}\]
done
clear
B)
\[B{{r}^{-}}\]
done
clear
C)
\[{{F}^{-}}\]
done
clear
D)
\[{{I}^{-}}\]
done
clear
View Answer play_arrow
question_answer 71) The concentration of \[[{{H}^{+}}]\] and concentration of \[[O{{H}^{-}}]\] of a 0.1 aqueous solution of 2% ionised weak acid is: [Ionic product of water =1 x 10~14]
A)
\[2\times {{10}^{-3}}M\] and \[5\times {{10}^{-12}}M\]
done
clear
B)
\[1\times {{10}^{3}}M\] and \[3\times {{10}^{-11}}M\]
done
clear
C)
\[0.02\times {{10}^{-3}}M\] and \[5\times {{10}^{-11}}M\]
done
clear
D)
\[3\times {{10}^{-2}}M\] and \[4\times {{10}^{-13}}M\]
done
clear
View Answer play_arrow
question_answer 72) 0.2 g of fine animal charcoal is mixed with half-litre of acetic acid solution and shaken for 30 min :
A)
concentration remains same
done
clear
B)
concentration increases
done
clear
C)
concentration of the solution decrease
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 73) A compound has a vapour density of 29. On warming an aqueous solution of alkali, it gives a yellow precipitate. The compound is:
A)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
B)
\[C{{H}_{3}}CHOHC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 74) Calculate de-Broglie wavelength of an electron travelling at 1% of the speed of light:
A)
\[2.73\times {{10}^{-24}}\]
done
clear
B)
\[2.42\times {{10}^{-10}}\]
done
clear
C)
\[242.2\times {{10}^{10}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 75) In the reaction \[HN{{O}_{3}}+{{P}_{4}}{{O}_{10}}\xrightarrow{{}}4HP{{O}_{3}}+X\], the product X is:
A)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 76) Which of the following information can be obtained on the basis of Le-Chateliers principle?
A)
Entropy change in a reaction
done
clear
B)
Equilibrium constant of a chemical reaction
done
clear
C)
Dissociation constant of weak acid
done
clear
D)
Shift in equilibrium position on changing value of a constant
done
clear
View Answer play_arrow
question_answer 77) One mole of NaCI (s) on melting absorbed 30.5 kJ of heat and its entropy is increased by 28.8 \[J{{K}^{-1}}\]. The melting point of NaCI is:
A)
1059 K
done
clear
B)
30.5 K
done
clear
C)
28.8 K
done
clear
D)
28800 K
done
clear
View Answer play_arrow
question_answer 78) Sulphur-35 (34.96903 amu) emits a \[\beta \] particle but no \[\gamma \]-rays, the product is chlorine-35 (34.96885 amu). The maximum energy emitted by the \[\beta \]-particle is :
A)
0.016767 MeV
done
clear
B)
1.6758 MeV
done
clear
C)
0.016758 MeV
done
clear
D)
16.758 MeV
done
clear
View Answer play_arrow
question_answer 79) At what pressure will a quantity of gas which occupies 100 mL at a pressure of 720 mm, occupy a volume of 84 mL?
A)
736.18mm
done
clear
B)
820.20mm
done
clear
C)
784.15mm
done
clear
D)
857.14mm
done
clear
View Answer play_arrow
question_answer 80) A coordination complex compound of cobalt has the molecular formulae containing five ammonia molecules, one nitro group and two chlorine atoms for one cobalt atom. One mole of this compound produces three mole ions in an aqueous solution on reacting this solution with excess of \[AgN{{O}_{3}},\,AgCl\] precipitate. The ionic formula for this complex would be :
A)
\[[Co{{(N{{H}_{3}})}_{5}}(N{{O}_{2}})]C{{l}_{2}}\]
done
clear
B)
\[[Co{{(N{{H}_{3}})}_{5}}Cl][Cl(N{{O}_{2}})]\]
done
clear
C)
\[[Co{{(N{{H}_{3}})}_{4}}(N{{O}_{2}})Cl][(N{{H}_{3}})Cl]\]
done
clear
D)
\[[Co{{(N{{H}_{3}})}_{5}}][{{(N{{O}_{2}})}_{2}}C{{l}_{2}}]\]
done
clear
View Answer play_arrow
question_answer 81) Iron pipes lying under acidic soil are often attached to blocks of magnesium for protection from rusting. Magnesium offers protection to iron against corrosion because it:
A)
prevents air from reaching the surface of iron
done
clear
B)
is more readily converted into positive ions
done
clear
C)
is heavier than iron
done
clear
D)
forms a corrosion-resistant alloy with iron
done
clear
View Answer play_arrow
question_answer 82) The dyes which are applied to the fabric in the colourless reduced state and then oxidised to coloured state are called :
A)
vat dyes
done
clear
B)
disperse dyes
done
clear
C)
triphenyl methane dye
done
clear
D)
azo dyes
done
clear
View Answer play_arrow
question_answer 83) In \[PO_{4}^{3-}\] ion, the formal charge on each oxygen atom and P?0 bond order respectively are :
A)
-0.75, 1.25
done
clear
B)
-0.75, 1.0
done
clear
C)
-0.75, 0.6
done
clear
D)
-3.1, 25
done
clear
View Answer play_arrow
question_answer 84) Which of the following alkali metal ions has lowest ionic mobility in aqueous solutions?
A)
\[R{{b}^{+}}\]
done
clear
B)
\[C{{s}^{+}}\]
done
clear
C)
\[L{{i}^{+}}\]
done
clear
D)
\[N{{a}^{+}}\]
done
clear
View Answer play_arrow
question_answer 85) The reason for double helical structure of DNA is operation of:
A)
electrostatic attractions
done
clear
B)
van der Waals forces
done
clear
C)
dipole-dipole interactions
done
clear
D)
hydrogen bonding
done
clear
View Answer play_arrow
question_answer 86) Bond order of \[{{O}_{2}}\] is:
A)
2
done
clear
B)
1.5
done
clear
C)
3
done
clear
D)
3.5
done
clear
View Answer play_arrow
question_answer 87) Which of the following statement is false?
A)
Photochemical smog causes irritation in eyes
done
clear
B)
London smog is a mixture of smoke and fog
done
clear
C)
Photochemical smog results in the formation of PAN
done
clear
D)
London smog is oxidising in nature
done
clear
View Answer play_arrow
question_answer 88) The total number of possible isomers of the complex compound \[[C{{u}^{II}}{{(N{{H}_{3}})}_{4}}][P{{t}^{II}}C{{l}_{4}}]\] is:
A)
3
done
clear
B)
6
done
clear
C)
5
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 89) A certain buffer solution contains equal concentrations of \[{{X}^{-1}}\] and HX. \[{{K}_{b}}\] for \[{{X}^{-}}\] is\[{{10}^{-10}}\]. The pH of the buffer solution is:
A)
4
done
clear
B)
10
done
clear
C)
7
done
clear
D)
14
done
clear
View Answer play_arrow
question_answer 90) The most efficient agent for the absorption of 503 is:
A)
90% \[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
98% \[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
80% \[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
20% \[{{H}_{2}}S{{O}_{7}}\]
done
clear
View Answer play_arrow
question_answer 91) Primary aldehyde on oxidation gives:
A)
esters
done
clear
B)
carboxylic acid
done
clear
C)
ketones
done
clear
D)
alcohols
done
clear
View Answer play_arrow
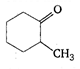
question_answer 92)
IUPAC name for the compound
A)
a-methyl cyclohexanone
done
clear
B)
2-methyl cyclohexanone
done
clear
C)
heptanone-2
done
clear
D)
methyl cyclo-hexanone
done
clear
View Answer play_arrow
question_answer 93) What is the volume of 0.1 \[N-HCl\] required to react completely with 1.0 g of pure calcium carbonate?
A)
100 \[c{{m}^{3}}\]
done
clear
B)
150 \[c{{m}^{3}}\]
done
clear
C)
250 \[c{{m}^{3}}\]
done
clear
D)
200 \[c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 94) \[C{{H}_{3}}COOH\] is reacted with \[CH\equiv CH\] in presence of \[H{{g}^{2+}}\], the product is:
A)
\[\underset{\begin{smallmatrix} | \\ C{{H}_{2}}(OOC{{H}_{3}}) \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}(OOCC{{H}_{3}})\]
done
clear
B)
\[\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}-(OOC-C{{H}_{3}})\]
done
clear
C)
\[\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,H{{(O\,OC-C{{H}_{3}})}_{2}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 95) The four quantum numbers of the valence electron of potassium are :
A)
4, 1, 0 and \[\frac{1}{2}\]
done
clear
B)
4, 0,1 and \[\frac{1}{2}\]
done
clear
C)
4, 0, 0 and \[+\frac{1}{2}\]
done
clear
D)
4, 1, 1, and \[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 96) \[CuS{{O}_{4}}\] reacts with KCN solution and forms :
A)
\[{{K}_{3}}[Cu{{(CN)}_{4}}]\]
done
clear
B)
\[Cu(CN)\]
done
clear
C)
\[Cu{{(CN)}_{2}}\]
done
clear
D)
\[{{K}_{4}}[Cu{{(CN)}_{6}}]\]
done
clear
View Answer play_arrow
question_answer 97) 1.00 g of a non-electrolyte solute dissolved in 50.5 g of benzene lowered the freezing point of benzene by 0.40 K. \[{{K}_{f}}\] for benzene is 5.12 kg \[mo{{l}^{-1}}\]. Molecular mass of the solute will be:
A)
256 g \[mo{{l}^{-1}}\]
done
clear
B)
2.56 g \[mo{{l}^{-1}}\]
done
clear
C)
\[512\times {{10}^{3}}g\,\,mo{{l}^{-1}}\]
done
clear
D)
\[2.56\times {{10}^{4}}g\,\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 98) \[C{{H}_{2}}=C{{H}_{2}}\xrightarrow{Br/{{H}_{2}}O}A\],In the above reaction the compound A is:
A)
ethylene bromohydrin
done
clear
B)
1, 2-dibromo ethane
done
clear
C)
ethanol
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 99) Which of the following will not undergo hydrolysis in water?
A)
Ammonium sulphate
done
clear
B)
Sodium sulphate
done
clear
C)
Copper sulphate
done
clear
D)
All the salts will hydrolyse
done
clear
View Answer play_arrow
question_answer 100) \[\underset{{{S}^{o}}(298K)\,J{{K}^{-1}}-10.7}{\mathop{{{H}^{+}}(aq)+O{{H}^{-}}(aq)}}\,\xrightarrow{{}}\underset{+70}{\mathop{{{H}_{2}}O\,\,(l)}}\,\] Standard entropy change for the above reaction is:
A)
\[60.3\,\,J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
B)
\[80.7\,\,J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
C)
\[-70.7\,\,J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
D)
\[+10.7\,\,J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 101) Cyanide resistant pathway is:
A)
anaerobic respiration
done
clear
B)
aerobic respiration
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 102) Which of the following is used for observing spindle fibres?
A)
Dark Held microscope
done
clear
B)
Phase contrast microscope
done
clear
C)
Polarisation microscope
done
clear
D)
Scanning transmission electron microscope
done
clear
View Answer play_arrow
question_answer 103) Following technique uses radioactive precursors for observing metabolic activities of macromolecules, is:
A)
chromatography
done
clear
B)
density gradient centrifugation or cell fractionation
done
clear
C)
autoradiography
done
clear
D)
electron microscope
done
clear
View Answer play_arrow
question_answer 104) Secondary cell wall grows by :
A)
deamination
done
clear
B)
calcicole
done
clear
C)
apposition
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 105) Single stranded DNA is found in :
A)
polio viruses
done
clear
B)
rice dwarf virus
done
clear
C)
TMV
done
clear
D)
\[\phi \] X174
done
clear
View Answer play_arrow
question_answer 106) Agranal chloroplast are found in :
A)
Bryophytes
done
clear
B)
Gymnospenns
done
clear
C)
Green algae
done
clear
D)
Angiosperms
done
clear
View Answer play_arrow
question_answer 107) Ribosomes that occur exclusively inm itochondria is :
A)
70S
done
clear
B)
55S
done
clear
C)
30S
done
clear
D)
50S
done
clear
View Answer play_arrow
question_answer 108) Number of carboxylation occur in Calvin cycle, is:
A)
0
done
clear
B)
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 109) R.Q. (Respiratory Quotient) is defined as :
A)
volume of \[C{{O}_{2}}\] evolved = volume of \[{{O}_{2}}\] consumed
done
clear
B)
\[\frac{\text{Volume}\,\text{of}\,{{\text{O}}_{\text{2}}}\,\text{consumed}}{\text{Volume}\,\text{of}\,\text{C}{{\text{O}}_{\text{2}}}\,\text{evolved}}\]
done
clear
C)
\[\frac{\text{Volume}\,\text{of}\,\text{C}{{\text{O}}_{\text{2}}}\,\text{evolved}}{\text{Volume}\,\text{of}\,{{\text{C}}_{\text{2}}}\,\text{consumed}}\]
done
clear
D)
\[\frac{\text{Volume}\,\text{of}\,{{\text{O}}_{\text{2}}}\,\text{evolved}}{\text{Volume}\,\text{of}\,\text{C}{{\text{O}}_{\text{2}}}\,\text{consumed}}\]
done
clear
View Answer play_arrow
question_answer 110) Pseudostratified epithelium is found in :
A)
pharynx
done
clear
B)
trachea
done
clear
C)
testis
done
clear
D)
epidermis
done
clear
View Answer play_arrow
question_answer 111) Activity of succinic dehydrogenase involves the following in TCA cycle :
A)
NAD
done
clear
B)
FAD
done
clear
C)
GDP
done
clear
D)
ATP
done
clear
View Answer play_arrow
question_answer 112) The process by which there is inhibition of aerobic respiration by atmospheric \[{{O}_{2}}\] is:
A)
Pasteurs effect
done
clear
B)
Calvins effect
done
clear
C)
Darwins effect
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 113) Decarboxylation is not involved in :
A)
electron transport system
done
clear
B)
\[{{C}_{4}}\] cycle
done
clear
C)
Krebs cycle
done
clear
D)
alcoholic fermentation
done
clear
View Answer play_arrow
question_answer 114) Which of the following is involved in the catalysis of link reaction during aerobic respiration?
A)
Vitamin-A
done
clear
B)
Vitamin-\[{{B}_{1}}\]
done
clear
C)
Vitamin-\[{{B}_{6}}\]
done
clear
D)
Vitamin-K
done
clear
View Answer play_arrow
question_answer 115) DNA and histone proteins are synthesized during the following phase of cell cycle :
A)
S-phase
done
clear
B)
\[{{G}_{2}}\]-phase
done
clear
C)
\[{{G}_{1}}\]-phase
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 116) Chromosomes that determine male sex in Melandrium plant is:
A)
Y chromosome
done
clear
B)
X chromosome
done
clear
C)
XX chromosome
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 117) Aqueduct of sylvius (iter) connects :
A)
1st and 2nd ventricles
done
clear
B)
3rd and 4th ventricles
done
clear
C)
2nd and 3rd ventricles
done
clear
D)
4th and 1st ventricles
done
clear
View Answer play_arrow
question_answer 118) Spores with chloroplast is present in :
A)
Selaginella
done
clear
B)
Equisetum
done
clear
C)
Puccinia
done
clear
D)
Rhizopus
done
clear
View Answer play_arrow
question_answer 119) Anabaena has an immense potential as a :
A)
biofertilizer
done
clear
B)
food
done
clear
C)
medicines
done
clear
D)
sewage disposal
done
clear
View Answer play_arrow
question_answer 120) Which of the following gymnosperm has the presence of vessel?
A)
Pinus
done
clear
B)
Ephedra
done
clear
C)
Cycas
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 121) Physiological heterospory is seen in :
A)
Chlamydomonas
done
clear
B)
Rhizopus
done
clear
C)
Selaginella
done
clear
D)
Lycopodium
done
clear
View Answer play_arrow
question_answer 122) Polyembryony is shown by :
A)
Selaginella
done
clear
B)
Pinus
done
clear
C)
Mangifera
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 123) Wood ofCycos is :
A)
pycnoxylic only
done
clear
B)
polyxylic only
done
clear
C)
manoxylic and diploxylic
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 124) The stele found in monocot is :
A)
haplostele
done
clear
B)
atactostele
done
clear
C)
dictyostele
done
clear
D)
actinostele
done
clear
View Answer play_arrow
question_answer 125) Smallest flower is:
A)
Wolffia microspermia
done
clear
B)
Salvinia
done
clear
C)
Pistfa
done
clear
D)
Lemna
done
clear
View Answer play_arrow
question_answer 126) Pseudocoelom is not found in :
A)
Ascaris
done
clear
B)
Ancylostoma
done
clear
C)
Fasdola
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 127) Integumentary nephridia are also called:
A)
enteronephric
done
clear
B)
exonephric
done
clear
C)
sometimes enteronephric and sometimes exonephric
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 128) Herdmania belongs to which subphyla :
A)
Cephalochordata
done
clear
B)
Hemichordata
done
clear
C)
Urochordata
done
clear
D)
Protochordata
done
clear
View Answer play_arrow
question_answer 129) Salmon is:
A)
anadromous fish
done
clear
B)
catadromous fish
done
clear
C)
mollusca
done
clear
D)
insect
done
clear
View Answer play_arrow
question_answer 130) Which of the following passage way is part of cloaca of vertebrates?
A)
Rectum
done
clear
B)
The reproductive tract
done
clear
C)
The urinary tract
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 131) One gene one enzyme hypothesis was given by:
A)
Jacob and Monod
done
clear
B)
McClintock
done
clear
C)
Meselson and Stahl
done
clear
D)
Beadle and Tatum
done
clear
View Answer play_arrow
question_answer 132) Ribosome mainly has :
A)
DNA
done
clear
B)
RNA
done
clear
C)
carbohydrate
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 133) Hybrid vigour is due to :
A)
heterosis
done
clear
B)
epistasis
done
clear
C)
complementary genes
done
clear
D)
supplementary genes
done
clear
View Answer play_arrow
question_answer 134) The phenotypic ratio obtained in quantitative inheritance of a dihybrid cross is :
A)
1 : 2 : 1
done
clear
B)
1 : 4 : 6 : 4 : 1
done
clear
C)
1 : 6 : 15 : 20 : 15 : 6: 1
done
clear
D)
9 : 3 : 3 : 1
done
clear
View Answer play_arrow
question_answer 135) In Limnaea shell coiling is due to :
A)
cytoplasmic inheritance
done
clear
B)
nuclear inheritance
done
clear
C)
recessive inheritance
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 136) Binomial nomenclature was given by:
A)
Aristotle
done
clear
B)
Hutchinson
done
clear
C)
Julian Huxley
done
clear
D)
Linnaeus
done
clear
View Answer play_arrow
question_answer 137) Vernalisation is response due to :
A)
high temperature
done
clear
B)
optimum temperature
done
clear
C)
low temperature
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 138) The number of chromosomes in Klinefelters syndrome is :
A)
47(44 + XXY)
done
clear
B)
47 (44 + XXX)
done
clear
C)
47 [46 + 1 (chromosome 21)]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 139) Oblique ovary is found in family :
A)
Brassicaceae
done
clear
B)
Compositae
done
clear
C)
Leguminosae
done
clear
D)
Solanaceae
done
clear
View Answer play_arrow
question_answer 140) Codes of m-RNA and proteins are :
A)
coplanar
done
clear
B)
colinear
done
clear
C)
nonlinear
done
clear
D)
irregular
done
clear
View Answer play_arrow
question_answer 141) Abnormal gene is replaced by normal gene through:
A)
gene therapy
done
clear
B)
medicines
done
clear
C)
cloning
done
clear
D)
radiation
done
clear
View Answer play_arrow
question_answer 142) Smallest part of DNA, which takes part in crossing over is called:
A)
gene
done
clear
B)
allele
done
clear
C)
recon
done
clear
D)
( d) none of these
done
clear
View Answer play_arrow
question_answer 143) Gibberellin was first reported from :
A)
algae
done
clear
B)
fungus
done
clear
C)
bacteria
done
clear
D)
bryophyte
done
clear
View Answer play_arrow
question_answer 144) Maximum photosynthetic activity takes place in:
A)
red light
done
clear
B)
orange light
done
clear
C)
green light
done
clear
D)
yellow light
done
clear
View Answer play_arrow
question_answer 145) Animals devoid of respiratory, excretory and circulatory organs are belong to phylum:
A)
Echinodermata
done
clear
B)
Platyhelminthes
done
clear
C)
Porifera
done
clear
D)
Mollusca
done
clear
View Answer play_arrow
question_answer 146) Ginger multiplies vegetatively by:
A)
tuber
done
clear
B)
corm
done
clear
C)
sucker
done
clear
D)
rhizome
done
clear
View Answer play_arrow
question_answer 147) In rabbit axis vertebrae is identified by :
A)
sigmoid notch
done
clear
B)
odontoblast
done
clear
C)
odontoid process
done
clear
D)
olecranon process
done
clear
View Answer play_arrow
question_answer 148) Trochophore larva is found in :
A)
Annelida
done
clear
B)
Platyhelminthes
done
clear
C)
Coelenterate
done
clear
D)
Porifera
done
clear
View Answer play_arrow
question_answer 149) 8th vertebrae of frog is :
A)
amphiplatyan
done
clear
B)
procoelous
done
clear
C)
amphicoelous
done
clear
D)
opisthocoelous
done
clear
View Answer play_arrow
question_answer 150) Larva form of housefly is called :
A)
wriggler
done
clear
B)
pupa
done
clear
C)
tumbler
done
clear
D)
maggot
done
clear
View Answer play_arrow
question_answer 151) Which of the following is absent in the segment of cockroachs leg?
A)
Fibula
done
clear
B)
Coxa
done
clear
C)
Tibia
done
clear
D)
Femur
done
clear
View Answer play_arrow
question_answer 152) which of the following belongs to class Insecta?
A)
Julus
done
clear
B)
Silver fish
done
clear
C)
Lobsters
done
clear
D)
Prawn
done
clear
View Answer play_arrow
question_answer 153) Soil formed after leaching and rich in Al and Fe is:
A)
alluvial
done
clear
B)
podsol
done
clear
C)
laterite
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 154) In micturition :
A)
urethra relaxes
done
clear
B)
ureter relaxes
done
clear
C)
ureter contracts
done
clear
D)
urethra contracts
done
clear
View Answer play_arrow
question_answer 155) Different species residing in different geographical areas are :
A)
allopatric
done
clear
B)
parapatric
done
clear
C)
sympatric
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 156) Stratification is most complex in :
A)
tropical forest
done
clear
B)
deciduous forest
done
clear
C)
savanna
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 157) Sporogony of malarial parasite occur in :
A)
liver of man
done
clear
B)
stomach wall of mosquito
done
clear
C)
RBCs of man
done
clear
D)
salivary gland of mosquito
done
clear
View Answer play_arrow
question_answer 158) Examples of regional pollution are :
A)
acid rain
done
clear
B)
smog
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 159) Single membrane bound organelles are :
A)
lysosome
done
clear
B)
sphaerosome
done
clear
C)
glyoxysome
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 160) The non-membranous organelles are :
A)
centrioles
done
clear
B)
ribosomes
done
clear
C)
nucleolus
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 161) Fossilization would occur when flora and fauna are burried by:
A)
natural processes
done
clear
B)
industrial processes
done
clear
C)
both [a] and [b]
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 162) \[N{{a}^{+}},{{K}^{+}}\] dependent ATP ase activity helps in transport of:
A)
\[{{K}^{+}}\] inward, \[N{{a}^{+}}\] outward
done
clear
B)
\[{{K}^{+}}\] inward only
done
clear
C)
\[N{{a}^{+}}\] inward only
done
clear
D)
\[{{K}^{+}}\] outward, \[N{{a}^{+}}\] inward
done
clear
View Answer play_arrow
question_answer 163) Which of the following mineral deficiency will cause death of stem and root tips?
A)
Mo
done
clear
B)
Ca
done
clear
C)
S
done
clear
D)
Fe
done
clear
View Answer play_arrow
question_answer 164) In submerged hydrophytes entry of \[C{{O}_{2}}\] is through :
A)
epidermis as dissolved \[C{{O}_{2}}\]
done
clear
B)
epidermis as carbonates only
done
clear
C)
epidermis as bicarbonates only
done
clear
D)
both [b] and [c]
done
clear
View Answer play_arrow
question_answer 165) Which lenses in electron microscope are used to control and focus a beam of electron?
A)
Convex lens
done
clear
B)
Concave lens
done
clear
C)
Electric lens
done
clear
D)
Magnetic lens
done
clear
View Answer play_arrow
question_answer 166) Damaged sieve tubes are sealed by deposition of:
A)
pectin
done
clear
B)
callus
done
clear
C)
suberin
done
clear
D)
lignin
done
clear
View Answer play_arrow
question_answer 167) Which of the following is a seed borne disease?
A)
Bacterial blight of rice
done
clear
B)
Kharia of paddy
done
clear
C)
Whiptail of Brassica
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 168) Vascular cambium in roots takes its origin from:
A)
pericycle
done
clear
B)
conjunctive parenchyma
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 169) Atropa belladona yields medicine used for :
A)
gastric ulcers
done
clear
B)
checking the eyes
done
clear
C)
leprosy
done
clear
D)
constipation
done
clear
View Answer play_arrow
question_answer 170) In monocot stem, following is absent:
A)
endodermis
done
clear
B)
hypodermis
done
clear
C)
cortex
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 171) One micrometer is a unit equivalent to :
A)
\[{{10}^{-3}}m\]
done
clear
B)
\[{{10}^{-6}}m\]
done
clear
C)
\[{{10}^{-9}}m\]
done
clear
D)
\[{{10}^{-12}}m\]
done
clear
View Answer play_arrow
question_answer 172) Juicy part of litchi is an outgrowth of:
A)
nucellus
done
clear
B)
micropyle end
done
clear
C)
chalazal end
done
clear
D)
outer integument
done
clear
View Answer play_arrow
question_answer 173) Which among the following is the only vertebrate osmoconformer?
A)
Rabbit
done
clear
B)
Hagfish
done
clear
C)
Bird
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 174) Removal of amino group of ammo acid to transform it into keto acid is :
A)
transamination
done
clear
B)
ammonification
done
clear
C)
deamination
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 175) The heartbeat of which animal is myogenic in nature?
A)
Cockroach
done
clear
B)
Leech
done
clear
C)
Elephant
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 176) The dental formula of rat is :
A)
\[\frac{0033}{3133}\]
done
clear
B)
\[\frac{1003}{1003}\]
done
clear
C)
\[\frac{2123}{2123}\]
done
clear
D)
\[\frac{3131}{3121}\]
done
clear
View Answer play_arrow
question_answer 177) Deltoid ridge is present in:
A)
femur
done
clear
B)
humerus
done
clear
C)
tibia
done
clear
D)
fibula
done
clear
View Answer play_arrow
question_answer 178) Connecting link between Annelida and Mollusca is :
A)
Neopilina
done
clear
B)
Nautilus
done
clear
C)
glochidium larva
done
clear
D)
veliger larva
done
clear
View Answer play_arrow
question_answer 179) Osphradium ofPlia globosa is :
A)
photoreceptor
done
clear
B)
thermoreceptor
done
clear
C)
chemoreceptor
done
clear
D)
tangoreceptor
done
clear
View Answer play_arrow
question_answer 180) Nissls granules are absent in :
A)
axon
done
clear
B)
cyton
done
clear
C)
dendrone
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 181) How many years ago abiogenesis occurred?
A)
3.5 billion years
done
clear
B)
3.0 billion years
done
clear
C)
2.5 billion years
done
clear
D)
3.2 billion years
done
clear
View Answer play_arrow
question_answer 182) Organs that have different embryonic origin but perform similar functions are :
A)
homologous organs
done
clear
B)
analogous organs
done
clear
C)
vestigial organs
done
clear
D)
atavism
done
clear
View Answer play_arrow
question_answer 183) Fossils are studied for :
A)
tracing evolutionary history of organisms
done
clear
B)
studying extinct organisms
done
clear
C)
providing jobs to scientist
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 184) In situ conservation of natural genetic resources can be achieved by establishing:
A)
national park
done
clear
B)
wild life sanctuaries
done
clear
C)
biosphere reserve
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 185) Last stable community in succession which dependent on climate is:
A)
seral community
done
clear
B)
climax community
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 186) Healing of wound in plants takes place by at activity of:
A)
ground tissue
done
clear
B)
callus deposition
done
clear
C)
secondary meristem
done
clear
D)
permanent tissue
done
clear
View Answer play_arrow
question_answer 187) Weed killers generally have properties much like that of:
A)
hormone
done
clear
B)
carbohydrate
done
clear
C)
enzymes
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 188) Which of the following inhibits \[{{O}_{2}}\] release light phase?
A)
PMA
done
clear
B)
Zeatin
done
clear
C)
DCMU
done
clear
D)
None-of these
done
clear
View Answer play_arrow
question_answer 189) Suspension of isolated thylakoids in culture medium containing \[C{{O}_{2}}\]and \[{{H}_{2}}O\] does produce hexose due to absence of which of the following?
A)
ATP
done
clear
B)
Enzyme
done
clear
C)
Proteins
done
clear
D)
Hill reagent
done
clear
View Answer play_arrow
question_answer 190) During absorption of \[{{H}_{2}}O\] by roots, the \[{{H}_{2}}O\]potential of cell sap is lower than that of:
A)
solution outside
done
clear
B)
pure \[{{H}_{2}}O\]
done
clear
C)
one
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 191) Jacket of fern antheridium is composed of:
A)
2 cells
done
clear
B)
3 cells
done
clear
C)
4 cells
done
clear
D)
5 cells
done
clear
View Answer play_arrow
question_answer 192) Triticum monococcum is :
A)
diploid
done
clear
B)
triploid
done
clear
C)
tetraploid
done
clear
D)
hexaploid
done
clear
View Answer play_arrow
question_answer 193) Hybrid breakdown refers to the condition when offspring are physiologically inferior to the following generation?
A)
\[{{F}_{1}}\]
done
clear
B)
\[{{F}_{2}}\]
done
clear
C)
\[{{P}_{1}}~\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 194) Which statement is correct?
A)
Osmotic pressure of solution is greater than pure solvent
done
clear
B)
Osmotic pressure of solution is lower than the pure water
done
clear
C)
Osmotic pressure of solution is equal to the pure water
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 195) During Ineiosis crossing over occur between which part of homologous chromosome?
A)
Sister chromatids
done
clear
B)
Nonsister chromatids
done
clear
C)
Genes
done
clear
D)
Alleles
done
clear
View Answer play_arrow
question_answer 196) The neuromuscular transmitter is:
A)
dopamine
done
clear
B)
epinephrine
done
clear
C)
norepinephrine
done
clear
D)
acetylcholine
done
clear
View Answer play_arrow
question_answer 197) Monosomy and trisomy are respectively:
A)
\[n-1,n+2\]
done
clear
B)
\[2n+2,2n+1\]
done
clear
C)
\[2n-1,2n+1\]
done
clear
D)
\[n-2,2n+1\]
done
clear
View Answer play_arrow
question_answer 198) In which phylum larva/young one is bilaterally symmetrical but adult one is radially symmetrical?
A)
Echinodermata
done
clear
B)
Porifera
done
clear
C)
Arthropoda
done
clear
D)
Mollusca
done
clear
View Answer play_arrow
question_answer 199) Tendons and ligaments are specialized types of:
A)
nervous tissue
done
clear
B)
muscular tissue
done
clear
C)
epithelial tissue
done
clear
D)
fibrous connective tissue
done
clear
View Answer play_arrow
question_answer 200) Diabetes mellitus takes place only when :
A)
\[\alpha \]-cells of pancreas are in excess
done
clear
B)
\[\text{ }\!\!\beta\!\!\text{ }\]-cells of pancreas are in excess
done
clear
C)
\[\alpha \]-cells of pancreas are in hypo
done
clear
D)
\[\text{ }\!\!\beta\!\!\text{ }\]-cells of pancreas are in hypo
done
clear
View Answer play_arrow
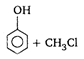
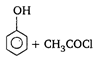
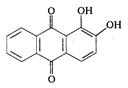
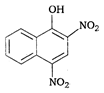
 is:
is:
