question_answer 1) Which of the following pairs does not have identical dimensions?
A)
Moment of inertia and moment of force
done
clear
B)
Impulse and momentum
done
clear
C)
Work and torque
done
clear
D)
Angular momentum and Plancks constant
done
clear
View Answer play_arrow
question_answer 2) If a body falling from rest described distance\[{{s}_{1,}}\]\[{{s}_{2,}}\]and.\[{{s}_{3,}}\] in the first, second and third seconds of its fall, then the ratio \[{{s}_{1,}}:\,{{s}_{2,}}:\,{{s}_{3,}}\]will be :
A)
1 : 4 : 9
done
clear
B)
1 : 3 : 5
done
clear
C)
1 : 2 : 3
done
clear
D)
1 : 1 : 1
done
clear
View Answer play_arrow
question_answer 3) A block of mass mi rests on a horizontal table. A string tied to this block is passed over a friction less pulley fixed at one end of the table and another block of mass \[{{m}_{2}}\] is hung to the other end of the string, the acceleration a of the system will be :
A)
\[\frac{{{m}_{1}}{{m}_{2}}g}{{{m}_{1}}-{{m}_{2}}}\]
done
clear
B)
\[\frac{{{m}_{2}}g}{{{m}_{1}}+{{m}_{2}}}\]
done
clear
C)
\[\frac{{{m}_{1}}g}{{{m}_{1}}+{{m}_{2}}}\]
done
clear
D)
\[{{m}_{1}}{{m}_{2}}g\]
done
clear
View Answer play_arrow
question_answer 4) The escape velocity of sphere of mass m from earths surface will be: (G = universal gravitational constant, \[{{M}_{e}}\]= mass of the earth, \[{{R}_{e}}\] = radius of the earth)
A)
\[\sqrt{\frac{2G{{M}_{e}}+{{R}_{e}}}{{{R}_{e}}}}\]
done
clear
B)
\[\sqrt{\frac{2G{{M}_{e}}m}{{{R}_{e}}}}\]
done
clear
C)
\[\sqrt{\frac{2G{{M}_{e}}}{{{R}_{e}}}}\]
done
clear
D)
\[\frac{G{{M}_{e}}}{{{R}_{e}}}\]
done
clear
View Answer play_arrow
question_answer 5) A force of 1000 N is applied on a body of mass 100 kg moving with the velocity of 5 m/s. How much time does it require to attain a velocity of 25 m/s?
A)
8 s
done
clear
B)
6 s
done
clear
C)
4 s
done
clear
D)
2 s
done
clear
View Answer play_arrow
question_answer 6) A cylinder of mass 10 kg is rolling on a plane with an initial velocity of 10 m/s. If the coefficient of friction between the surface and the cylinder is 0.5, then before stopping it will cover a distance of; \[(g=10\,m/{{s}^{2}})\]
A)
2.5m
done
clear
B)
0.5m
done
clear
C)
7.5m
done
clear
D)
10m
done
clear
View Answer play_arrow
question_answer 7) Two springs of spring constants 1000 N/m and 4000 N/m respectively are stretched with the same force, their potential energies will be in ratio:
A)
1 : 2
done
clear
B)
4 : 1
done
clear
C)
1 : 4
done
clear
D)
16 : 1
done
clear
View Answer play_arrow
question_answer 8) The readings of a constant-volume gas thermometer at \[{{0}^{o}}C\] and \[{{100}^{o}}C\] are 40 cm of mercury column and 60 cm of mercury column respectively. If its reading at an unknown temperature is 100 cm of mercury column, then find that temperature.
A)
\[{{200}^{o}}C\]
done
clear
B)
\[{{300}^{o}}C\]
done
clear
C)
\[{{500}^{o}}C\]
done
clear
D)
\[{{150}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 9) The disc of a siren has n holes and the frequency of its rotation is 300 rpm. It produces a note of wavelength 2.4 m. If the velocity of sound in air is 360 m/s, then the value of n will be:
A)
30
done
clear
B)
34
done
clear
C)
12
done
clear
D)
15
done
clear
View Answer play_arrow
question_answer 10) At which temperature, the velocity of sound will be four times of its velocity at \[{{0}^{o}}C\]?
A)
\[{{8322}^{o}}C\]
done
clear
B)
\[{{3822}^{o}}C\]
done
clear
C)
\[{{1911}^{o}}C\]
done
clear
D)
\[{{4095}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 11) A particle of mass m moving with the velocity\[v\] collides with a stationary particle of mass 2m and sticks to it. The speed of the system after collision is :
A)
\[3v\]
done
clear
B)
\[\frac{1}{3}v\]
done
clear
C)
\[2v\]
done
clear
D)
\[\frac{1}{2}v\]
done
clear
View Answer play_arrow
question_answer 12) Resolve a weight of 10 N in two directions which are parallel and perpendicular to a slope inclined at \[{{30}^{o}}\] to the horizontal.
A)
\[{{w}_{\bot }}=5\sqrt{3}N,\,{{w}_{||}}=5N\]
done
clear
B)
\[{{w}_{\bot }}=5N,\,{{w}_{||}}=5\sqrt{3}N\]
done
clear
C)
\[{{w}_{\bot }}=5\sqrt{3},\,{{w}_{||}}=0N\]
done
clear
D)
\[{{w}_{\bot }}=0N,\,{{w}_{||}}=5N\]
done
clear
View Answer play_arrow
question_answer 13) A car accelerates from rest at a constant rate \[\alpha \] for some time, after which it decelerates at a constant rate \[\beta \] and comes to rest. When total elapsed time is t, then the maximum velocity acquired by the car is :
A)
\[\frac{({{\alpha }^{2}}+{{\beta }^{2}})t}{\alpha \,\beta }\]
done
clear
B)
\[\frac{({{\alpha }^{2}}-{{\beta }^{2}})t}{\alpha \,\beta }\]
done
clear
C)
\[\frac{(\alpha +\beta )t}{\alpha \,\beta }\]
done
clear
D)
\[\frac{\alpha \beta t}{\alpha \,+\beta }\]
done
clear
View Answer play_arrow
question_answer 14) A body freely falling from the rest has a velocity v after it falls through a height h. The distance, covered by body when it falls down with double of velocity, is :
A)
\[12\,h\]
done
clear
B)
\[8\,h\]
done
clear
C)
\[9\,h\]
done
clear
D)
\[4\,h\]
done
clear
View Answer play_arrow
question_answer 15) A train weighing \[{{10}^{7}}N\] is running on a level track with uniform velocity of 36 km/h. The frictional force is \[0.5\times {{10}^{3}}N\]. What is the power of the engine? \[(g=10\,m/{{s}^{2}})\]
A)
50 kW
done
clear
B)
500 kW
done
clear
C)
0.5 kW
done
clear
D)
5kW
done
clear
View Answer play_arrow
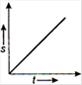
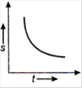
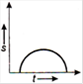
question_answer 16) Which one of the following graphs shows uniformly accelerated motion?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 17) The door of a domestic refrigerator is left open while the switch is on. Then the room will:
A)
remain at same temperature
done
clear
B)
get heated
done
clear
C)
get cooled
done
clear
D)
first [b] and then [c]
done
clear
View Answer play_arrow
question_answer 18) If kinetic energy of a body becomes four times, then its new momentum will:
A)
become four times its initial value
done
clear
B)
become two times its initial value
done
clear
C)
become half of its initial value
done
clear
D)
remain constant
done
clear
View Answer play_arrow
question_answer 19) Two simple pendulums A and B of time periods 2 s and 2.1s are made to vibrate simultaneously. They are initially in phase. After how many vibrations, they will be in the same phase?
A)
35
done
clear
B)
30
done
clear
C)
25
done
clear
D)
21
done
clear
View Answer play_arrow
question_answer 20) A mass m attached to a spring oscillates with time period 2 s. If the mass is increased by 2 kg, then time period increases by 1 s. Then the initial mass of the body will be :
A)
12.8kg
done
clear
B)
8.6kg
done
clear
C)
1.6kg
done
clear
D)
3.2kg
done
clear
View Answer play_arrow
question_answer 21) Two identical balls A and B collide head on elastically. If velocities of A and B before the collision are + 0.5 m/s and - 0.3 m/s respectively, then their velocities after the collision will be :
A)
+ 0.5nysand+ 0.3 m/s
done
clear
B)
- 0.5 m/s and + 0.3 m/s
done
clear
C)
+ 0.3 m/s and - 0.5 m/s
done
clear
D)
- 0.3 m/s and + 0.5 m/s
done
clear
View Answer play_arrow
question_answer 22) The electric intensity due to a dipole of length 10 cm and having a charge of \[500\,\mu C\] at a point on the axis at a distance 20 cm from one of the charges in air is :
A)
\[20.5\times {{10}^{7}}N/C\]
done
clear
B)
\[18.28\,\,N/C\]
done
clear
C)
\[6.25\times {{10}^{7}}N/C\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 23) If the potential of a capacitor having capacity \[8\,\mu F\] is increased from 15V to 25 V, then increase in its energy is :
A)
\[12\times {{10}^{-6}}J\]
done
clear
B)
\[16\times {{10}^{-4}}J\]
done
clear
C)
\[8\times {{10}^{-6}}J\]
done
clear
D)
\[4\times {{10}^{-4}}J\]
done
clear
View Answer play_arrow
question_answer 24) In a transformer, number of turns of primary coil and secondary coil are 5 and 4, respectively. If 220 V is applied in primary coil, then the ratio of the current in primary and secondary coils will be :
A)
5 : 8
done
clear
B)
8 : 5
done
clear
C)
4 : 5
done
clear
D)
5 : 4
done
clear
View Answer play_arrow
question_answer 25) A particle having charge 100 times that of an electron is revolving in a circular path of radius. 0.8 m with 1 rot/s. The magnetic field produced at the center will be :
A)
\[{{10}^{-17}}\,{{\mu }_{0}}\]
done
clear
B)
\[{{10}^{-14}}\,{{\mu }_{0}}\]
done
clear
C)
\[{{10}^{-7}}\,{{\mu }_{0}}\]
done
clear
D)
\[{{10}^{-5}}\,{{\mu }_{0}}\]
done
clear
View Answer play_arrow
question_answer 26) Electron beam is allowed to pass normally through mutually perpendicular electric field and magnetic field. If magnetic field induction and electric field strength are 0.0005 \[Wb/{{m}^{2}}\]and 4000 V/m respectively, then the beam suffers no deflection. The velocity of electron will be :
A)
\[16\times {{10}^{8}}m/s\]
done
clear
B)
\[8.0\times {{10}^{6}}m/s\]
done
clear
C)
\[4.0\times {{10}^{6}}m/s\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 27) When a metal with work function 0.8eV is illuminated with light of 3eV, then the stopping potential will be:
A)
3.3eV
done
clear
B)
1.1eV
done
clear
C)
2.2eV
done
clear
D)
none of there
done
clear
View Answer play_arrow
question_answer 28) The work function of a photo metal is 6.626 eV. Its threshold wavelength will be :
A)
\[1873\,\overset{o}{\mathop{A}}\,\]
done
clear
B)
\[1675\,\overset{o}{\mathop{A}}\,\]
done
clear
C)
\[2875\,\overset{o}{\mathop{A}}\,\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 29) A prism of refracting angle \[{{60}^{o}}\] is made with a material of refractive index\[\,\mu \]. For a certain wavelength of light, the angle of minimum deviation is \[{{30}^{o}}\]. For this wavelength the value of \[\,\mu ,\] the material will be :
A)
1.531
done
clear
B)
1.414
done
clear
C)
1.593
done
clear
D)
1.825
done
clear
View Answer play_arrow
question_answer 30) A source of light is placed at 10 cm from a convex mirror and is then moved upon a distance of 2 cm from the mirror. How much does the image move, if the radius of curvature of the mirror is 4.8 cm?
A)
0.95cm
done
clear
B)
0.85cm
done
clear
C)
0.75cm
done
clear
D)
None of these
done
clear
View Answer play_arrow
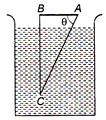
question_answer 31)
A glass prism of refractive index 1.5 is A immersed in water of refractive index \[\frac{4}{3.}\]A light incident normally on the face AB is totally reflected to reach the face AC, if :
A)
\[\sin \,\theta \,>\frac{3}{2}\]
done
clear
B)
\[\sin \,\theta \,>\frac{2}{3}\]
done
clear
C)
\[\sin \,\theta \,>\frac{8}{9}\]
done
clear
D)
\[\frac{2}{3}\sin \,\theta \,>\frac{8}{9}\]
done
clear
View Answer play_arrow
question_answer 32) When a current of 0.2 A is drawn from a battery, then potential difference between its terminals is 20 V and when a current of 2 A is drawn, then the potential difference drops to 16 V. The emf of battery will be :
A)
20.4eV
done
clear
B)
24.4eV
done
clear
C)
19.8eV
done
clear
D)
16.1eV
done
clear
View Answer play_arrow
question_answer 33) A black body at a high temperature T Kelvin radiates energy at the rate of E\[watt/{{m}^{2}}\]. When T the temperature falls to \[\frac{T}{2}\] kelvin, then the radiated energy is :
A)
\[\frac{E}{16}\]
done
clear
B)
\[2E\]
done
clear
C)
\[\frac{E}{4}\]
done
clear
D)
\[\frac{E}{8}\]
done
clear
View Answer play_arrow
question_answer 34) Consider a hypothetical annihilation of a stationary electron with a stationary position. What is the wavelength of resulting radiation?
A)
\[\frac{hc}{{{m}_{0}}}\]
done
clear
B)
\[\frac{h}{{{m}_{0}}c}\]
done
clear
C)
\[\frac{h}{{{m}_{0}}{{c}^{2}}}\]
done
clear
D)
\[\frac{{{m}_{0}}c}{h}\]
done
clear
View Answer play_arrow
question_answer 35) Minimum excitation potential of Bohrs first orbit in hydrogen atom is :
A)
3.4eV
done
clear
B)
3.6eV
done
clear
C)
10.2eV
done
clear
D)
13.6eV
done
clear
View Answer play_arrow
question_answer 36) The \[6563\,\overset{o}{\mathop{A}}\,\] line emitted by hydrogen atom in a star is found to be red shifted by 15 A. The speed with which the star is receding from the earth is :
A)
\[9.66\times {{10}^{9}}m/s\]
done
clear
B)
\[6.86\times {{10}^{5}}m/s\]
done
clear
C)
\[7.78\times {{10}^{5}}m/s\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 37) What is the voltage gain in a common-emitter amplifier where input resistance is 3 0 and load resistance is \[24\,\Omega \]? \[(\beta =0.6)\]
A)
2.2
done
clear
B)
1.2
done
clear
C)
4.8
done
clear
D)
8.16
done
clear
View Answer play_arrow
question_answer 38) The wavelength of \[{{k}_{\alpha }}\] X-rays produced by a X ray tube is \[0.76\,\overset{o}{\mathop{A}}\,\]. What is the atomic number of the anode material of the tube?
A)
20
done
clear
B)
29
done
clear
C)
40
done
clear
D)
59
done
clear
View Answer play_arrow
question_answer 39) If red light and violet light have the focal lengths \[{{f}_{r}}\] and\[{{f}_{v}}\]respectively, then which one of the following is correct?
A)
\[{{\lambda }_{r}}\le {{\lambda }_{v}}\]
done
clear
B)
\[{{\mu }_{r}}>{{\mu }_{v}}\]
done
clear
C)
\[{{\mu }_{r}}<{{\mu }_{v}}\]
done
clear
D)
\[{{\lambda }_{r}}>{{\lambda }_{v}}\]
done
clear
View Answer play_arrow
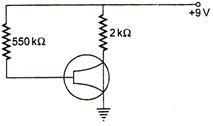
question_answer 40)
The figure shows an n-p-n transistor in common-emitter configuration. If\[{{I}_{C}}=1.5\,mA,\,\,\beta =100\], then value of \[{{V}_{BC}}\] is:
A)
-5.25V
done
clear
B)
-0.75V
done
clear
C)
6V
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 41) In a capacitor or capacitance \[20\,\mu F,\] the distance between the plates is 2 mm. If a dielectric of width 1 mm and dielectric constant 2 is inserted between the plates, then new capacitance will be :
A)
\[32\,\mu F\]
done
clear
B)
\[26.7\,\mu F\]
done
clear
C)
\[13.3\,\mu F\]
done
clear
D)
\[3\,\mu F\]
done
clear
View Answer play_arrow
question_answer 42) The time taken by the light to cross a glass of thickness 4 mm and refractive index \[(\mu =3)\] is:
A)
\[8\times {{10}^{-10}}s\]
done
clear
B)
\[16\times {{10}^{-11}}s\]
done
clear
C)
\[2\times {{10}^{-11}}s\]
done
clear
D)
\[4\times {{10}^{-11}}s\]
done
clear
View Answer play_arrow
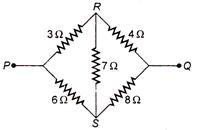
question_answer 43)
In the given figure the equivalent resistance between P and Q is :
A)
\[\frac{14}{9}\Omega \]
done
clear
B)
\[\frac{9}{14}\Omega \]
done
clear
C)
\[\frac{3}{14}\Omega \]
done
clear
D)
\[\frac{14}{3}\Omega \]
done
clear
View Answer play_arrow
question_answer 44) If the mass of the earth is 80 times that of moon and its radius is two times that of the moon, and g on the earth is \[9.8\,m/{{s}^{2}}\], then value of g on the moon will be :
A)
\[4.9\,m/{{s}^{2}}\]
done
clear
B)
\[0.49\,m/{{s}^{2}}\]
done
clear
C)
\[0.98\,m/{{s}^{2}}\]
done
clear
D)
\[9.8\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 45) A volume of a gas at \[{{20}^{o}}C\] is \[100\,c{{m}^{3}}\]at normal pressure. If it is heated to \[{{100}^{o}}C\] its volume becomes 125 cm at the same pressure the volume coefficient of the gas will be normal pressure) :
A)
\[0.0021{{/}^{o}}C\]
done
clear
B)
\[0.0025{{/}^{o}}C\]
done
clear
C)
\[0.0030{{/}^{o}}C\]
done
clear
D)
\[0.0033{{/}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 46) Two waves of length 50 cm and 51 cm produce 12 beats/s, the velocity of sound will be :
A)
360 m/s
done
clear
B)
340 m/s
done
clear
C)
331 m/s
done
clear
D)
306 m/s
done
clear
View Answer play_arrow
question_answer 47) Two closed organ pipes when sounded simultaneously give 4 beats/s. If longer tube has a length of 1 m, then length of shorter pipe will be (v = 330 m/s ):
A)
104.8cm
done
clear
B)
95.4cm
done
clear
C)
104cm
done
clear
D)
96cm
done
clear
View Answer play_arrow
question_answer 48)
Four capacitors are connected as shown.
A)
\[30.6\mu F\]
done
clear
B)
\[20.33\mu F\]
done
clear
C)
\[6.67\mu F\]
done
clear
D)
\[3.33\mu F\]
done
clear
View Answer play_arrow
question_answer 49) The ratio of Rydbergs constant for helium to the Rydbergs constant for hydrogen is :
A)
1 : 2
done
clear
B)
1 : 4
done
clear
C)
4 : 1
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 50) If the distance between the two charged particles are doubled, then the force will :
A)
remain same
done
clear
B)
be one-fourth
done
clear
C)
be halved
done
clear
D)
be doubled
done
clear
View Answer play_arrow
question_answer 51) Producer gas is a mixture of:
A)
\[CO+{{N}_{2}}\]
done
clear
B)
\[CO+{{H}_{2}}\]
done
clear
C)
\[Ca+Fe\]
done
clear
D)
\[CO+S\]
done
clear
View Answer play_arrow
question_answer 52) Which one of the following metal is obtained by electrolytic reduction?
A)
Fe
done
clear
B)
Cu
done
clear
C)
Ag
done
clear
D)
Al
done
clear
View Answer play_arrow
question_answer 53) The molecule which has pyramidal shape is :
A)
\[PC{{l}_{3}}\]
done
clear
B)
\[S{{O}_{3}}\]
done
clear
C)
\[CO_{3}^{2-}\]
done
clear
D)
\[NO_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 54) The bond dissociation energies of \[{{H}_{2}},C{{l}_{2}}\] and \[HCl\] are 104, 58 and 103 kcal respectively. The enthalpy of formation of \[HCl\] gas will be:
A)
-44 kcal
done
clear
B)
-88 kcal
done
clear
C)
-22kcal
done
clear
D)
-11 kcal
done
clear
View Answer play_arrow
question_answer 55) The IUPAC name of \[C{{H}_{3}}-\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,}}\,-C{{H}_{2}}-\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}\] is:
A)
4- methyl-2, 4-pentanediol
done
clear
B)
2-methyl-2, 4-pentanediol
done
clear
C)
1, 1-dimethyl 1, 3-butanediol
done
clear
D)
1, 2 3- trimethyl-1, 3-propanediol
done
clear
View Answer play_arrow
question_answer 56) Entropy of vaporisation of water at \[{{100}^{o}}C\], if molar heat of vaporisation is 9710 cal \[mo{{l}^{-1}}\], will be:
A)
20 cal \[mo{{l}^{-1}}{{K}^{-1}}\]
done
clear
B)
26 cal \[mo{{l}^{-1}}{{K}^{-1}}\]
done
clear
C)
24 cal \[mo{{l}^{-1}}{{K}^{-1}}\]
done
clear
D)
28 cal \[mo{{l}^{-1}}{{K}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 57) An electron having highest energy in the set:
A)
3, 2, 1, \[\frac{1}{2}\]
done
clear
B)
4, 2, - 1, \[\frac{1}{2}\]
done
clear
C)
4, 1, 0, \[-\frac{1}{2}\]
done
clear
D)
5, 0, 0, \[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 58) \[{{C}_{6}}{{H}_{5}}N{{H}_{2}}+CHC{{l}_{3}}+KOH\xrightarrow{{}}?\]
A)
phenyl isocyanide
done
clear
B)
benzyl amine
done
clear
C)
benzyl chloride
done
clear
D)
ethyl iso-cyanide
done
clear
View Answer play_arrow
question_answer 59) The nitrogen atoms has 7 electrons, the nitride ion \[({{N}^{3-}})\] will have:
A)
4 protons and 7 electrons
done
clear
B)
7 protons and 10 electrons
done
clear
C)
4 protons and 10 electrons
done
clear
D)
10 protons and 7 electrons
done
clear
View Answer play_arrow
question_answer 60) The momentum of a particle with de-Broglies wavelength of 6A is :
A)
\[1.1\times {{10}^{-24}}kg\,m{{s}^{-1}}\]
done
clear
B)
\[1.1\times {{10}^{-34}}kg\,m{{s}^{-1}}\]
done
clear
C)
\[39.6\times {{10}^{-34}}kg\,m{{s}^{-1}}\]
done
clear
D)
\[39.6\times {{10}^{-24}}kg\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 61) The volume of 2.8 g of CO at \[{{27}^{o}}C\] and 0.821 atm pressure is: (R = 0.0821 L atm \[mol\,{{s}^{-1}}{{K}^{-1}}\])
A)
30 mL
done
clear
B)
3L
done
clear
C)
0.3 L
done
clear
D)
1.5 L
done
clear
View Answer play_arrow
question_answer 62) When primary alcohol is oxidized with chlorine, it gives:
A)
\[C{{l}_{3}}HO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[CC{{l}_{3}}CHO\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 63) The size of ionic species is correctly given in order:
A)
\[C{{l}^{7+}}>S{{i}^{4+}}>M{{g}^{2+}}>N{{a}^{+}}\]
done
clear
B)
\[N{{a}^{+}}>M{{g}^{2+}}>S{{i}^{4+}}>C{{l}^{7+}}\]
done
clear
C)
\[N{{a}^{+}}>M{{g}^{2+}}>C{{l}^{7+}}>S{{i}^{4+}}\]
done
clear
D)
\[C{{l}^{7+}}>N{{a}^{+}}>M{{g}^{2+}}>S{{i}^{4+}}\]
done
clear
View Answer play_arrow
question_answer 64) Oxidation number of Ni in \[Ni{{(CO)}_{4}}\] is:
A)
2
done
clear
B)
3
done
clear
C)
1
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 65) If the volume of 2 moles of an ideal gas at 540 K is 44.8 L. Then its pressure will be:
A)
1 atm
done
clear
B)
2 atm
done
clear
C)
3 atm
done
clear
D)
4 atm
done
clear
View Answer play_arrow
question_answer 66) One mole of \[C{{O}_{2}}\] contain:
A)
\[6.02\times {{10}^{23}}\] atoms of C
done
clear
B)
\[6.02\times {{10}^{23}}\] atoms of O
done
clear
C)
\[18.1\times {{10}^{23}}\] atoms of \[C{{O}_{2}}\]
done
clear
D)
3 g-atoms of \[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 67) A compound with empirical formula \[C{{H}_{2}}O\]has a vapour density of 30. Its molecular formula is:
A)
\[C{{H}_{2}}O\]
done
clear
B)
\[{{C}_{2}}{{H}_{4}}{{O}_{2}}\]
done
clear
C)
\[{{C}_{3}}{{H}_{6}}{{O}_{3}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\]
done
clear
View Answer play_arrow
question_answer 68) When acetamide reacts with \[B{{r}_{2}}\] in presence of \[NaOH\], there is a formation of:
A)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}CN\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
D)
\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 69) Frenkel defect is shown by :
A)
\[AgBr\]
done
clear
B)
ZnS
done
clear
C)
\[Agl\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 70) The atomic size from left to right in periodic table:
A)
increases
done
clear
B)
decreases
done
clear
C)
no change
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 71) Which molecule is not linear?
A)
\[C{{O}_{2}}\]
done
clear
B)
\[S{{O}_{3}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
D)
\[MgC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 72) The correct order of increasing ionic character is:
A)
\[BeC{{l}_{2}}<CaC{{l}_{2}}<MgC{{l}_{2}}<BaC{{l}_{2}}\]
done
clear
B)
\[BeC{{l}_{2}}<MgC{{l}_{2}}<CaC{{l}_{2}}<BaC{{l}_{2}}\]
done
clear
C)
\[BeC{{l}_{2}}<BaC{{l}_{2}}<MgC{{l}_{2}}<CaC{{l}_{2}}\]
done
clear
D)
\[BaC{{l}_{2}}<CaC{{l}_{2}}<MgC{{l}_{2}}<BeC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 73) Glass reacts with HF giving:
A)
\[{{F}_{2}}\]
done
clear
B)
\[{{H}_{2}}Si{{F}_{6}}\]
done
clear
C)
\[Ca{{F}_{2}}\]
done
clear
D)
\[NaF\]
done
clear
View Answer play_arrow
question_answer 74) Two molecules of an aldehyde react with concentrated solution of caustic soda and produce one molecule of an alcohol and acid each. The aldehyde is:
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 75) A fee crystal contains how many atoms in each unit cell?
A)
4
done
clear
B)
6
done
clear
C)
3
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 76) The bond order is maximum in:
A)
\[{{H}_{2}}\]
done
clear
B)
\[H_{2}^{+}\]
done
clear
C)
\[H{{e}_{2}}\]
done
clear
D)
\[H{{e}_{2}}^{+}\]
done
clear
View Answer play_arrow
question_answer 77) Oxidation of acetaldehyde with selenium dioxide produces:
A)
glyoxal
done
clear
B)
oxalic acid
done
clear
C)
ethanoic acid
done
clear
D)
methanoic acid
done
clear
View Answer play_arrow
question_answer 78) Which is not emitted by radioactive substance?
A)
\[\alpha \]-rays
done
clear
B)
\[\beta \]-rays
done
clear
C)
Positron
done
clear
D)
Proton
done
clear
View Answer play_arrow
question_answer 79) Which of the following is not a colligative property?
A)
\[\Delta {{T}_{f}}\]
done
clear
B)
\[\pi \]
done
clear
C)
\[\Delta {{T}_{b}}\]
done
clear
D)
\[{{K}_{b}}\]
done
clear
View Answer play_arrow
question_answer 80) The equivalent weight of iron in \[F{{e}_{2}}{{O}_{3}}\] would be:
A)
18.6
done
clear
B)
22.66
done
clear
C)
56
done
clear
D)
112
done
clear
View Answer play_arrow
question_answer 81) Gold number is the index for:
A)
protective colloid
done
clear
B)
purity of gold
done
clear
C)
metallic gold
done
clear
D)
electroplated gold
done
clear
View Answer play_arrow
question_answer 82)
Arrange the following in order of increasing C - C bond length?
(i) \[{{C}_{6}}{{H}_{6}}\]
(ii) \[CH\equiv CH\]
(iii) \[{{H}_{3}}C-C{{H}_{3}}\]
(iv) \[{{H}_{2}}C-C{{H}_{2}}\]
A)
\[I>II>III>IV\]
done
clear
B)
\[II>IV>I>III\]
done
clear
C)
\[III>I>IV>II\]
done
clear
D)
\[III>IV>II>I\]
done
clear
View Answer play_arrow
question_answer 83) \[{{K}_{p}}/{{K}_{c}}\] for the reaction \[CO(g)+\frac{1}{2}{{O}_{2}}(g)C{{O}_{2}}(g)\] is:
A)
1
done
clear
B)
\[\frac{1}{4}\]
done
clear
C)
\[\frac{1}{\sqrt{RT}}\]
done
clear
D)
\[{{[RT]}^{1/2}}\]
done
clear
View Answer play_arrow
question_answer 84) Half-life period of a substance is 1600 min. How much fraction of the substance will remain after 6400 min?
A)
\[\frac{1}{16}\]
done
clear
B)
\[\frac{1}{4}\]
done
clear
C)
\[\frac{1}{8}\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 85) For a reaction \[4A+5B4P+6Q\], the equilibrium constant \[{{K}_{c}}\] has the units:
A)
\[[mol\,{{L}^{-1}}]\]
done
clear
B)
\[[mo{{l}^{-1}}\,L]\]
done
clear
C)
\[{{[mol\,{{L}^{-1}}]}^{-2}}\]
done
clear
D)
unitless
done
clear
View Answer play_arrow
question_answer 86) A solution of Baeyers reagent:
A)
contains cold dilute \[CuC{{l}_{2}}\] and \[HCl\]
done
clear
B)
contains cold dilute alk. \[KMn{{O}_{4}}\] solution
done
clear
C)
can be used for reduction purpose
done
clear
D)
can be used to detect unsaturation in phenol .
done
clear
View Answer play_arrow
question_answer 87) Collision theory satisfactorily explains:
A)
first order reaction
done
clear
B)
zero order reaction
done
clear
C)
bimolecular reaction
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 88) Plot of log (a - x) against time t is straight line. The reaction is of:
A)
second order
done
clear
B)
first order
done
clear
C)
zero order
done
clear
D)
third order
done
clear
View Answer play_arrow
question_answer 89) The weight of a single atom of oxygen is :
A)
\[0.057\times {{10}^{23}}g\]
done
clear
B)
\[2.657\times {{10}^{-23}}g\]
done
clear
C)
\[2.657\times {{10}^{23}}g\]
done
clear
D)
\[4.657\times {{10}^{-23}}g\]
done
clear
View Answer play_arrow
question_answer 90) A first order reaction is 75% complete after 32 min. When was 50% of the reaction completed?
A)
16 min
done
clear
B)
8 min
done
clear
C)
4 min
done
clear
D)
32 min
done
clear
View Answer play_arrow
question_answer 91) A biological catalyst is essentially :
A)
anaminoacid
done
clear
B)
a carbohydrate
done
clear
C)
an enzyme
done
clear
D)
a nitrogen molecule
done
clear
View Answer play_arrow
question_answer 92) Propane is obtained from propene by which of the following methods?
A)
Wurtz reaction
done
clear
B)
Dehydrogenation
done
clear
C)
Frankland reaction
done
clear
D)
Catalytic hydrogenation
done
clear
View Answer play_arrow
question_answer 93) The standard emf of the cell\[Zn+C{{u}^{2+}}\to Cu+Z{{n}^{2+}}\] is 1.10 at \[{{25}^{o}}C\]. The emf of the cell when 0.1M \[C{{u}^{2+}}\]and 0.1 M \[Z{{n}^{2+}}\] solution are used will be:
A)
1.10 V
done
clear
B)
+ 0.110 V
done
clear
C)
-1.10 V
done
clear
D)
-0.11 V
done
clear
View Answer play_arrow
question_answer 94) At \[{{90}^{o}}C\] pure water has\[{{H}_{3}}{{O}^{+}}={{10}^{-6}}mol/{{L}^{-1}}\]. The value of \[{{K}_{w}}\] at \[{{90}^{o}}C\] is:
A)
\[{{10}^{-6}}\]
done
clear
B)
\[{{10}^{-12}}\]
done
clear
C)
\[{{10}^{-14}}\]
done
clear
D)
\[{{10}^{-8}}\]
done
clear
View Answer play_arrow
question_answer 95) Degree of dissociation of 0.1 N \[C{{H}_{3}}COOH\]is: (dissociation constant \[=1\times {{10}^{-5}}\])
A)
\[{{10}^{-5}}\]
done
clear
B)
\[{{10}^{-4}}\]
done
clear
C)
\[{{10}^{-3}}\]
done
clear
D)
\[{{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 96) The standard cell potential of the galvanic cell, \[Zn/Z{{n}^{2+}}||A{{g}^{+}}/Ag\] where \[{{E}^{o}}_{Z{{n}^{2+}},Zn}=-0.76\,\,V\]and \[{{E}^{o}}_{A{{g}^{+}},Ag}=+0.80\,\,V\] is:
A)
+ 0.04V
done
clear
B)
-0.04V
done
clear
C)
+1.54 V
done
clear
D)
-1.54V
done
clear
View Answer play_arrow
question_answer 97) The solubility of \[AgCl\] is 0.0015 g/L. The solubility product of \[AgCl\] will be:
A)
\[2\times {{10}^{-10}}\]
done
clear
B)
\[1.1\times {{10}^{-10}}\]
done
clear
C)
\[3.1\times {{10}^{-10}}\]
done
clear
D)
\[4.1\times {{10}^{-10}}\]
done
clear
View Answer play_arrow
question_answer 98) Which of the following is formed by the action of water on sodium peroxide?
A)
\[{{H}_{2}}\]
done
clear
B)
\[{{N}_{2}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[CO\]
done
clear
View Answer play_arrow
question_answer 99) If 0.15 g of a solute dissolved in 15 g of solvent is boiled at a temperature higher by\[{{0.216}^{o}}C\], than that of the pure solvent, the molecular weight of the substance is: (Molal elevation constant for the solvent is\[{{216}^{o}}C\])
A)
10
done
clear
B)
10.1
done
clear
C)
100
done
clear
D)
1.01
done
clear
View Answer play_arrow
question_answer 100) Proteins are composed of:
A)
a amino acids
done
clear
B)
carbohydrates
done
clear
C)
vitamins
done
clear
D)
mineral salts
done
clear
View Answer play_arrow
question_answer 101) Veliger larva is found in the phylum :
A)
Mollusca
done
clear
B)
Echinodermata
done
clear
C)
Arthropoda
done
clear
D)
Cnidaria
done
clear
View Answer play_arrow
question_answer 102) Cyanide resistant respiration is characteristic of:
A)
viruses
done
clear
B)
bacteria
done
clear
C)
fungi
done
clear
D)
plants
done
clear
View Answer play_arrow
question_answer 103) In DNA helix cytosine is paired with guanine by:
A)
covalent bond
done
clear
B)
phosphate bond
done
clear
C)
two \[{{H}_{2}}\] bonds
done
clear
D)
three \[{{H}_{2}}\] bonds
done
clear
View Answer play_arrow
question_answer 104) ADP contains :
A)
one high energy bond
done
clear
B)
two high energy bonds
done
clear
C)
three high energy bonds
done
clear
D)
four energy bonds
done
clear
View Answer play_arrow
question_answer 105) The infective stage of Plasmodium is :
A)
sporozoite
done
clear
B)
trophozoite
done
clear
C)
merozoite
done
clear
D)
schizont
done
clear
View Answer play_arrow
question_answer 106) Reteroviruses have :
A)
only RNA as genetic material
done
clear
B)
only DNA as genetic material
done
clear
C)
DNA and RNA both
done
clear
D)
genes on nucleoprotein complexes as genetic material
done
clear
View Answer play_arrow
question_answer 107) Quill feathers at the base of quill wings are called:
A)
remiges
done
clear
B)
barbules
done
clear
C)
coverts
done
clear
D)
down feathers
done
clear
View Answer play_arrow
question_answer 108) Which of the following is an enterocoelic invertebrate ?
A)
Sepia
done
clear
B)
Cimex
done
clear
C)
Ophiothrix
done
clear
D)
Polygordius
done
clear
View Answer play_arrow
question_answer 109) Rajaji national park is in :
A)
Karnataka
done
clear
B)
Rajasthan
done
clear
C)
Uttarakhand
done
clear
D)
Assam
done
clear
View Answer play_arrow
question_answer 110) Sinus venosus is present in :
A)
birds only
done
clear
B)
birds and mammals
done
clear
C)
reptiles and birds
done
clear
D)
fishes, amphibians and reptiles
done
clear
View Answer play_arrow
question_answer 111) Coelom derived from blastocoel is known as:
A)
haemocoel
done
clear
B)
entero coelom
done
clear
C)
pseudocoelom
done
clear
D)
schizocoel
done
clear
View Answer play_arrow
question_answer 112) Which of the following cell type is capable of giving rise to other cell types in sponges ?
A)
Thesocytes
done
clear
B)
Pinacocytes
done
clear
C)
Collencytes
done
clear
D)
Archaeocytes
done
clear
View Answer play_arrow
question_answer 113) The function of peroxisomes is :
A)
\[{{H}_{2}}{{O}_{2}}\] destruction
done
clear
B)
conversion of fats to carbohydrates
done
clear
C)
detoxification of heavy metals
done
clear
D)
oxidation phosphorylation
done
clear
View Answer play_arrow
question_answer 114) Downs syndrome is characterised by :
A)
21st trisomy
done
clear
B)
two X and one Y chromosomes
done
clear
C)
19 trisomy
done
clear
D)
only one X chromosome
done
clear
View Answer play_arrow
question_answer 115) Which of the following is colourless Hydra ?
A)
Hydraused
done
clear
B)
Hydra viridis
done
clear
C)
Hydra oligactis
done
clear
D)
Hydra vulgaris
done
clear
View Answer play_arrow
question_answer 116) Predation and parasitism are which type of in teractions ?
A)
++
done
clear
B)
+-0
done
clear
C)
--
done
clear
D)
+-
done
clear
View Answer play_arrow
question_answer 117) Eggs of egg laying (prototherians) mammals are:
A)
macroledthal
done
clear
B)
telolecithal
done
clear
C)
alecithal
done
clear
D)
mesolecithal
done
clear
View Answer play_arrow
question_answer 118) The function of glyoxysome is :
A)
protein metabolism
done
clear
B)
carbohydrate metabolism
done
clear
C)
fat metabolism
done
clear
D)
protein synthesis
done
clear
View Answer play_arrow
question_answer 119) Gynaecophoric canal is found in :
A)
Taenia sagginata (male)
done
clear
B)
Schistosoma haematobium (male)
done
clear
C)
Wuchereria. bancrofti (male)
done
clear
D)
E. granulosus (female)
done
clear
View Answer play_arrow
question_answer 120) The excretory material of bony fish is :
A)
urea
done
clear
B)
protein
done
clear
C)
ammonia
done
clear
D)
amino acid
done
clear
View Answer play_arrow
question_answer 121) Cranial nerves numbering IV, V, VI are :
A)
trochlear, trigeminal, abducens
done
clear
B)
trochlear, trigeminal, facial
done
clear
C)
auditory, facial, trochlear
done
clear
D)
auditory, trochlear, facial
done
clear
View Answer play_arrow
question_answer 122) Cells which are secretory in function have abundant:
A)
lysosomes
done
clear
B)
E.R.
done
clear
C)
dictyosomes
done
clear
D)
osteosomes
done
clear
View Answer play_arrow
question_answer 123) Gonadotropins are secreted from :
A)
gonads
done
clear
B)
post pituitary
done
clear
C)
anterior pituitary
done
clear
D)
hypothalamus
done
clear
View Answer play_arrow
question_answer 124) Tasmanian wolf is a marsupial while wolf is a placental mammal this shows :
A)
convergent evolution
done
clear
B)
divergent evolution
done
clear
C)
parallelism
done
clear
D)
inheritance of acquired characters
done
clear
View Answer play_arrow
question_answer 125) Unwinding of DNA is done by :
A)
topoisomerase
done
clear
B)
exonuclease
done
clear
C)
helicase
done
clear
D)
ligase
done
clear
View Answer play_arrow
question_answer 126) When an animal has both the characters of male and female it is called :
A)
intersex
done
clear
B)
super male
done
clear
C)
super female
done
clear
D)
gynandromorphy
done
clear
View Answer play_arrow
question_answer 127) Insulin is secreted by :
A)
spleen
done
clear
B)
\[\text{ }\!\!\beta\!\!\text{ }\]-cells of pancreas
done
clear
C)
\[\alpha \]-cells of pancreas
done
clear
D)
mucosa of oesophagus
done
clear
View Answer play_arrow
question_answer 128) Book lungs are respiratory organs of:
A)
Mollusca
done
clear
B)
mammals
done
clear
C)
Arachnida
done
clear
D)
earthworm
done
clear
View Answer play_arrow
question_answer 129) Micturition reflex is related to :
A)
urination
done
clear
B)
ovulation
done
clear
C)
copulation
done
clear
D)
earthworm
done
clear
View Answer play_arrow
question_answer 130) One gene one enzyme theory was given by :
A)
Temin and Baltimore
done
clear
B)
Watson and Crick
done
clear
C)
Robert and Koch
done
clear
D)
Beadle and Tatum.
done
clear
View Answer play_arrow
question_answer 131) DNA synthesis occurs in.:
A)
prometaphase
done
clear
B)
prophase
done
clear
C)
interphase
done
clear
D)
telophase
done
clear
View Answer play_arrow
question_answer 132) Fat is converted into fatty acid and glycerol by enzyme :
A)
amylase
done
clear
B)
lipase
done
clear
C)
ptyalin
done
clear
D)
trypsin
done
clear
View Answer play_arrow
question_answer 133) In ruminants bacterial action takes place in :
A)
rumen
done
clear
B)
reticulum
done
clear
C)
omasum
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 134) Which of the following is a motor nerve ?
A)
Optic
done
clear
B)
Abducens
done
clear
C)
Auditory
done
clear
D)
Trigeminal nerve
done
clear
View Answer play_arrow
question_answer 135) The immediate cause of induction of ovulation in human female is large plasma surge of :
A)
LH
done
clear
B)
FSH
done
clear
C)
estrodiol
done
clear
D)
progesterone
done
clear
View Answer play_arrow
question_answer 136) Formation of RNA from DNA is known as :
A)
transcription
done
clear
B)
translation
done
clear
C)
replication
done
clear
D)
recombination
done
clear
View Answer play_arrow
question_answer 137) In the 28th day human ovarian cycle, me ovulation takes place typically on :
A)
1st day of the cycle
done
clear
B)
5th day of the cycle
done
clear
C)
14th day of the cycle
done
clear
D)
28th day of the cycle
done
clear
View Answer play_arrow
question_answer 138) Lungs are enclosed in :
A)
pericardium
done
clear
B)
peritonium
done
clear
C)
perichondrium
done
clear
D)
pleural membrane
done
clear
View Answer play_arrow
question_answer 139) Confusion technique involves the use of:
A)
juvenile hormone
done
clear
B)
pheromones
done
clear
C)
ecdysomes
done
clear
D)
hormones
done
clear
View Answer play_arrow
question_answer 140) Ontogeny recapitulates phylogeny. This is :
A)
Pauling law
done
clear
B)
Hardey-Weinberg law
done
clear
C)
Biogenetic law
done
clear
D)
Thomas law
done
clear
View Answer play_arrow
question_answer 141) A cell is heterozygous at three gene loci, how many different type of gamete can it form ?
A)
2
done
clear
B)
3
done
clear
C)
6
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 142) Which of the following is the most toxic excretory product ?
A)
\[C{{O}_{2}}\]
done
clear
B)
Urea
done
clear
C)
Ammonia
done
clear
D)
Ammo acids
done
clear
View Answer play_arrow
question_answer 143) Which of the following is concerned with the formation of urea in rabbit ?
A)
Blood
done
clear
B)
Kidney
done
clear
C)
Spleen
done
clear
D)
Liver
done
clear
View Answer play_arrow
question_answer 144) Lamarck was :
A)
French botanist who late become zoologist
done
clear
B)
English naturalist (gave theory of evolution)
done
clear
C)
French scientist (inheritance of acquired characters)
done
clear
D)
French scientist (law of inheritance)
done
clear
View Answer play_arrow
question_answer 145) 5th cranial nerve of frog is :
A)
optic
done
clear
B)
vagus
done
clear
C)
trigeminal
done
clear
D)
ophthalmic
done
clear
View Answer play_arrow
question_answer 146) Cancer of prostrate gland is caused due to exposure of:
A)
hydrocarbons
done
clear
B)
cadmium oxide
done
clear
C)
methane gas
done
clear
D)
strontium compound
done
clear
View Answer play_arrow
question_answer 147) Externally observable characters of an individual are called :
A)
phenotype
done
clear
B)
genotype
done
clear
C)
homozygous
done
clear
D)
heterozygous
done
clear
View Answer play_arrow
question_answer 148) Menstrual flow occurs due to lack of hormone:
A)
progesterone
done
clear
B)
FSH
done
clear
C)
oxytocin
done
clear
D)
vasopressin
done
clear
View Answer play_arrow
question_answer 149) Indian species of silkworm is:
A)
Bombyx mori
done
clear
B)
Apaphia
done
clear
C)
Aroylei
done
clear
D)
Aosscaman
done
clear
View Answer play_arrow
question_answer 150) Mongoloid condition is related with:
A)
Nullisomy
done
clear
B)
monosomy
done
clear
C)
trisomy
done
clear
D)
autosomy
done
clear
View Answer play_arrow
question_answer 151) The number of hydrogen bonds between adenine and thymine in DNA molecule are:
A)
Two
done
clear
B)
three
done
clear
C)
four
done
clear
D)
eight
done
clear
View Answer play_arrow
question_answer 152) Tetradynamous stamens are found in family:
A)
Malvaceae
done
clear
B)
Solanaceae
done
clear
C)
Cruciferae
done
clear
D)
Liliaceae
done
clear
View Answer play_arrow
question_answer 153) White rust disease is caused by:
A)
Claviceps
done
clear
B)
Alternaria
done
clear
C)
Phytophthora
done
clear
D)
Albugo Candida
done
clear
View Answer play_arrow
question_answer 154) The ecosystem of pond is referred as:
A)
lotic
done
clear
B)
lentic
done
clear
C)
xeric
done
clear
D)
benthic
done
clear
View Answer play_arrow
question_answer 155) The 10% energy transfer law of food chain was given by:
A)
Stanley
done
clear
B)
Tansley
done
clear
C)
Lindemann
done
clear
D)
Weismann
done
clear
View Answer play_arrow
question_answer 156) Heterosis is also called :
A)
mutation
done
clear
B)
variation
done
clear
C)
hybrid vigour
done
clear
D)
hybrid sterility
done
clear
View Answer play_arrow
question_answer 157) Hybrid vigour was discovered by :
A)
Jordon
done
clear
B)
Koelreuter
done
clear
C)
Shull
done
clear
D)
Johanson
done
clear
View Answer play_arrow
question_answer 158) Thigmotrophic movement is best shown by :
A)
movement in roots
done
clear
B)
insectivorous plants
done
clear
C)
movement in tendril
done
clear
D)
movement in Mimosa pudica
done
clear
View Answer play_arrow
question_answer 159) The latest trend in plant disease control is :
A)
chemical control
done
clear
B)
biological control
done
clear
C)
use of fertilizers
done
clear
D)
use of disease resistant varieties
done
clear
View Answer play_arrow
question_answer 160) In plants, peroxisomes are associated with :
A)
phototropism
done
clear
B)
photoperiodism
done
clear
C)
photosynthesis
done
clear
D)
Photorespiration
done
clear
View Answer play_arrow
question_answer 161) The function of rough endoplasmic reticulum is synthesis of:
A)
fat
done
clear
B)
lipid
done
clear
C)
protein
done
clear
D)
steroid
done
clear
View Answer play_arrow
question_answer 162) Which of the following plant kingdom, is called amphibians?
A)
Bryophyta
done
clear
B)
Trachaeophyta
done
clear
C)
Pteridophyta
done
clear
D)
Thallophyta
done
clear
View Answer play_arrow
question_answer 163) Which of the following plant virus has DNA is it?
A)
Tobacco mosaic virus
done
clear
B)
Tomato mosaic virus
done
clear
C)
Potato mosaic virus
done
clear
D)
Cauliflower mosaic virus
done
clear
View Answer play_arrow
question_answer 164) Dicotyledonous leaf showing parallel venation belongs to the genus :
A)
Pisum
done
clear
B)
Lathyrus
done
clear
C)
Physalia
done
clear
D)
Calotropis
done
clear
View Answer play_arrow
question_answer 165) A thin film of water is held by the soil particles under the influence of internal attractive force it is called:
A)
capillary water
done
clear
B)
combined water
done
clear
C)
hygroscopic water
done
clear
D)
gravitational water
done
clear
View Answer play_arrow
question_answer 166) Opium is obtained, from :
A)
Oryza sativa
done
clear
B)
Coffea arabica
done
clear
C)
Thea sinensis
done
clear
D)
Papaver somniferum
done
clear
View Answer play_arrow
question_answer 167) The process of mating of individuals, which are more closely related then the average of the population to which they belong, is called?
A)
Inbreeding
done
clear
B)
Hybridization
done
clear
C)
Heterosis
done
clear
D)
Self breeding
done
clear
View Answer play_arrow
question_answer 168) The nuclease enzyme, which begins its attack from free end of a polynucleotide, is?
A)
Exonuclease
done
clear
B)
Kinase
done
clear
C)
Polymerase
done
clear
D)
Endonuclease
done
clear
View Answer play_arrow
question_answer 169) Humus is:
A)
dead and shady organic matter
done
clear
B)
living organic and inorganic things
done
clear
C)
accumulation of radioactive materials
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 170) The periderm includes :
A)
cork
done
clear
B)
cambium
done
clear
C)
secondary cortex
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 171) A pigment which absorbs red and far red light is:
A)
carotene
done
clear
B)
xanthophyll
done
clear
C)
phytochrome
done
clear
D)
cytochrome
done
clear
View Answer play_arrow
question_answer 172) The chemical compounds produced by the host plants to protect themselves, against fungal infection is :
A)
phytotoxin
done
clear
B)
pathogen
done
clear
C)
phytoalexins
done
clear
D)
hormone
done
clear
View Answer play_arrow
question_answer 173) Parachute mechanism of seed dispersal occurs in :
A)
cotton
done
clear
B)
sunflower
done
clear
C)
Physalia
done
clear
D)
Calotropis
done
clear
View Answer play_arrow
question_answer 174) Energy enters in ecosystem through :
A)
herbivores
done
clear
B)
carnivores
done
clear
C)
producers
done
clear
D)
decomposers
done
clear
View Answer play_arrow
question_answer 175) ABA is involved in :
A)
root elongation
done
clear
B)
shoot elongation
done
clear
C)
dormancy of seeds
done
clear
D)
increased cell division
done
clear
View Answer play_arrow
question_answer 176) Gene therapy involves in :
A)
introducing of a normal genes in cell
done
clear
B)
eliminating defective and useless genes
done
clear
C)
treating of defective genes with radiations
done
clear
D)
replacement of defective genes by normal ones
done
clear
View Answer play_arrow
question_answer 177) Which of the following tissue helps in the flow of water in vascular plant?
A)
Xylem
done
clear
B)
Phloem
done
clear
C)
Cambium
done
clear
D)
Sieve tube cell
done
clear
View Answer play_arrow
question_answer 178) During light reaction of photosynthesis which of the following phenomenon is observed during cyclic phosphorylation as well as non-cyclic phosphorylation :
A)
release of \[{{O}_{2}}\]
done
clear
B)
formation of ATP
done
clear
C)
formation of NADPH
done
clear
D)
involvement of PS-I and PS-II
done
clear
View Answer play_arrow
question_answer 179) Phragmoplast is the precursor of :
A)
cell plate
done
clear
B)
chloroplast
done
clear
C)
chromoplast
done
clear
D)
colourless plastid
done
clear
View Answer play_arrow
question_answer 180) Conjugation occurs in :
A)
Funaria
done
clear
B)
Spirogyra
done
clear
C)
Oryza
done
clear
D)
Selaginella
done
clear
View Answer play_arrow
question_answer 181) The abundance of a species population, with in its habitat is called :
A)
niche density
done
clear
B)
absolute density
done
clear
C)
relative density
done
clear
D)
regional density
done
clear
View Answer play_arrow
question_answer 182) Respiratory quotient (R.Q.) during early stages of germination of castor seed is :
A)
zero
done
clear
B)
one
done
clear
C)
less than one
done
clear
D)
more than one
done
clear
View Answer play_arrow
question_answer 183) Maximum transpiration occurs in :
A)
algal cells
done
clear
B)
xerophytic plants
done
clear
C)
mesophytic plants
done
clear
D)
hydrophytic plants
done
clear
View Answer play_arrow
question_answer 184) Plasmids are :
A)
nucleoid
done
clear
B)
new type nurcoorganism
done
clear
C)
linear chromosome
done
clear
D)
extra chromosomal circular material
done
clear
View Answer play_arrow
question_answer 185) Nitrates are converted into nitrogen by :
A)
denitrifying bacteria
done
clear
B)
nitrogen fixing bacteria
done
clear
C)
carbon fixing bacteria
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 186) Which of the following pair is a sedimentary type of biogeochemical cycle?
A)
Oxygen and nitrogen
done
clear
B)
Phosphorus and sulphur
done
clear
C)
Phosphorus and nitrogen
done
clear
D)
Phosphorus and carbon dioxide
done
clear
View Answer play_arrow
question_answer 187) In which of the following stages the chromosomes appear thin, long and thread like?
A)
Zygotene
done
clear
B)
Pachytene
done
clear
C)
Leptotene
done
clear
D)
Diakinesis
done
clear
View Answer play_arrow
question_answer 188) Longitudinal duality of each chromosome of a homologous pair becomes clearly evident showing formation of four chromatids from each bivalent at;
A)
diplotene
done
clear
B)
zygotene
done
clear
C)
pachytene
done
clear
D)
diakinesis
done
clear
View Answer play_arrow
question_answer 189) The witches broom of legume is caused by :
A)
virus
done
clear
B)
bacteria
done
clear
C)
fungus
done
clear
D)
mycoplasma
done
clear
View Answer play_arrow
question_answer 190) The two great industrial tragedies namely MIC and Charnobyl respectively occurred where and at which time :
A)
Bhopal 1980, Ukrain 1990
done
clear
B)
Bhopal 1986, Ukrain 1988
done
clear
C)
Bhopal 1984, Ukrain 1986
done
clear
D)
Bhopal 1986, Russia 1988
done
clear
View Answer play_arrow
question_answer 191) The organism, used for alcohol fermentation, is:
A)
Pseudomonas
done
clear
B)
Penidllium
done
clear
C)
Aspergillus
done
clear
D)
Saccharomyces
done
clear
View Answer play_arrow
question_answer 192) The chloroplast of algae usually lack :
A)
grana
done
clear
B)
pigments
done
clear
C)
lamellae
done
clear
D)
quantasome
done
clear
View Answer play_arrow
question_answer 193) Haustorial root is present in :
A)
Zea mays
done
clear
B)
Cactus
done
clear
C)
Cuscuta
done
clear
D)
Orchid
done
clear
View Answer play_arrow
question_answer 194) Which of the following ion is involved in the closing and opening of stomata?
A)
\[M{{g}^{2+}}\]
done
clear
B)
\[N{{a}^{+}}\]
done
clear
C)
\[F{{e}^{2+}}\]
done
clear
D)
\[{{K}^{+}}\]
done
clear
View Answer play_arrow
question_answer 195) Chloroplasts, with pyrenoid like structures are found in the leaves of:
A)
Funaria
done
clear
B)
Cycos
done
clear
C)
Selaginella
done
clear
D)
Zea mays
done
clear
View Answer play_arrow
question_answer 196) Genes are responsible for the growth and differentiation in an organism through regulation of:
A)
translocation
done
clear
B)
transformation
done
clear
C)
transduction and translation
done
clear
D)
translation and transcription
done
clear
View Answer play_arrow
question_answer 197) As a tree grows older, which of the following increases more rapidly in thickness :
A)
phloem
done
clear
B)
cortex
done
clear
C)
heart wood
done
clear
D)
sap wood
done
clear
View Answer play_arrow
question_answer 198) Spore in Funaria, on germination gives :
A)
protonema
done
clear
B)
archegonia
done
clear
C)
antheridia
done
clear
D)
vegetative body
done
clear
View Answer play_arrow
question_answer 199) Green house-effect is due to :
A)
\[C{{O}_{2}}\]
done
clear
B)
\[CO\]
done
clear
C)
\[N{{O}_{2}}\]
done
clear
D)
\[P{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 200) Normally DNA molecule has A-T, G-C pairing. However, these bases can exist in alternative valency status, owing to rearrangement called?
A)
Point mutation
done
clear
B)
Analog substitution
done
clear
C)
Frame shift mutation
done
clear
D)
Tautomerisational mutation
done
clear
View Answer play_arrow

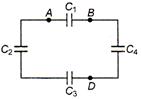 Each capacitor is of \[5\,\mu F\]. The equivalent capacitance between A and B is:
Each capacitor is of \[5\,\mu F\]. The equivalent capacitance between A and B is:
